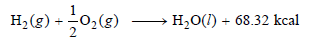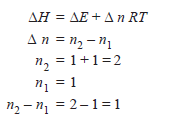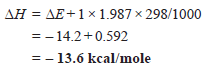Enthalpy of A System
– Enthalpy (H) is the total heat content of a system at constant pressure and is equivalent to the internal energy E plus the PV energy.
Enthalpy of A System
– In a process carried at constant volume (say in a sealed tube), the heat content of a system is the same as internal energy (E), as no PV work is done.
– But in a constant-pressure process, the system (a gas) also expends energy in doing PV work.
– Therefore, the total heat content of a system at constant pressure is equivalent to the internal energy E plus the PV energy.
– This is called the Enthalpy (Greek en = in; thalpos = heat) of the system and is represented by the symbol H.
– Enthalpy (H) is the total heat content of a system at constant pressure and is equivalent to the internal energy E plus the PV energy.
– Thus enthalpy is defined by the equation:
H = E + PV …(1)
Enthalpy – A Function of State
– In equation (1) above, E, P, and V are all state functions.
– Thus H, the value of which depends on the values of E, P, and V must also be a function of state.
– Hence its value is independent of the path by which the state of the system is changed.
Change in Enthalpy
– If ΔH is the difference in enthalpy of a system in the final state (H2) and that in the initial state (H1),
ΔH = H2 – H1 …(2)
– Substituting the values of H2 and H1, as from (1) and (2), we have:
ΔH = (E2 + P2V2) – (E1 + P1V1)
= (E2– E1) + (P2V2 – P1V1)
= ΔE + ΔPV
– If P is constant while the gas is expanding, we can write:
ΔH = ΔE + PΔV
ΔH = ΔE + w (w = work) …(3)
– According to the First Law,
ΔE = q – w …(4)
where q = heat transferred
– From equations (3) and (4):
ΔH = q
when the change in state occurs at constant pressure
– This relationship is usually written as:
ΔH = qp
where subscript p means constant pressure.
– Thus ΔH can be measured by measuring the heat of a process occurring at constant pressure.
Units and Sign Conventions of Enthalpy
– Since
ΔH = H2 – H1
– When ΔH is positive if H2 > H1 and the process or reaction will be endothermic.
– ΔH is negative if H1 > H2 and the reaction will be exothermic.
– In case of a chemical reaction carried in the laboratory in an open vessel:
ΔH = H products – H reactants = qp
– The heat of reaction at one atmosphere pressure is usually shown along with the equation. Thus,
– The quantity of heat 68.32 kcal on the right hand represents – ΔH of the reaction.
– The units of ΔH are kilocalories (kcal) or kilojoules (kJ).
Relation Between ΔH and ΔE
– The calorific values of many gaseous fuels are determined in constant volume calorimeters.
– These values are, therefore, given by the expression:
qv= ΔE
– When any fuel is burnt in the open atmosphere, additional energy of expansion, positive or negative, against the atmosphere is also involved.
– The value of q thus actually realized, i.e., qp = ΔH, may be different from the equation:
ΔH = ΔE + PΔV …(1)
– If gases are involved in a reaction, they account for most of the volume change as the volumes of solids and liquids are negligibly small in comparison.
– Suppose we have n1 moles of gases before the reaction, and n2 moles of gases after it.
– Assuming ideal gas behavior, we have:
P V2= n2 RT
P V1= n1 RT
∴ P (V2 – V1) = (n2 – n1) RT
PΔV = Δn RT
– Substituting in equation (1) we have,
ΔH = ΔE + Δn RT
Solved Problem
For the reaction:
Calculate ΔH for the reaction.
Solution:
Reference: Essentials of Physical Chemistry /Arun Bahl, B.S Bahl and G.D. Tuli / multicolour edition.