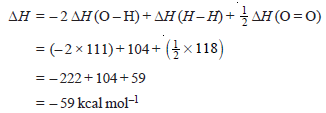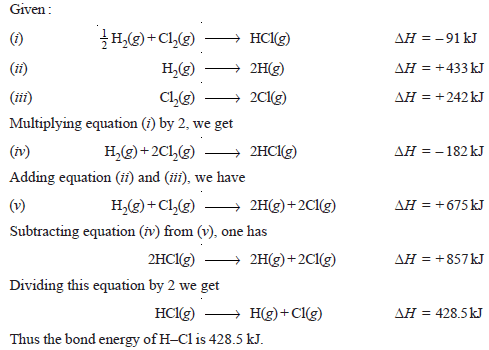Bond energy (definition, Illustration, solved Problems)
Bond energy
– When a bond between two atoms is formed, there is a release of energy.
– The same amount of energy is absorbed when the bond is broken.
– The bond energy is defined as the average amount of energy required to break all bonds of a particular type in one mole of the substance.
– Thus the bond energy of H – H bond is the energy required to break all the bonds in one mole of the gas.
– The bond energy is expressed in kcal mol–1 or kJ mol–1.
– For example, the bond energy of H – H bond is 433 kJ mol–1 or 103.58 kcal mol–1.
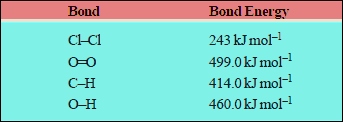
– The bond energies of some common bonds are listed below:
Bond Energy is a measure of the Strength of the Bond
– In other words, bond energy is the force with which the atoms are bonded together.
– It depends upon :
(1) Size of the atom
(3) Bond length
– A knowledge of bond enthalpy is useful for calculating heats of reaction for gaseous reactions for which no thermal data is available and which involve substances having covalent bonds.
– Suppose we desire to determine the bond energy of C–H bond in methane.
– For this purpose we need to know the enthalpy change for the reaction:
C(g) + 4H(g) → CH4(g)
– This is obtained by combining the heat of formation of methane from C(s) + H2(g) with the heat of sublimation of carbon i.e., C(s) → C(g) and the heat of dissociation of hydrogen into atoms i.e., H2(g) → 2H(g), which have been determined by spectroscopic methods.
– The value so obtained is 398 kcal mol–1 (or 1663.64 kJ mol–1).
– This represents the bond energy of four C–H bonds.
– Since all the bonds in methane are identical, the bond energy of C–H bond is 398/4 = 99.5 kcal mol–1.
– In a similar manner the bond energies of other types of bonds have been calculated.
– When a bond is broken, the bond energy is positive because heat is absorbed.
– It is written with a minus sign when a bond is formed and heat is evolved.
– The calculation of heat of reaction with the help of bond energies is illustrated in the following examples.
Solved Problem
Problem (1): Given that energies for H–H, O=O and O–H bonds are 104, 118 and 111 kcal mol–1 respectively, calculate the heat of the reaction:
H2(g) + ½ O2(g) → H2O(g)
Solution:
– In this reaction, two O–H bonds are formed and one H–H bond is broken.
– Therefore we can write for ΔH
– The heat of the given reaction is – 59.0 kcal mol–1
Problem (2): Calculate the bond energy of HCl, given that H–H bond energy is 433 kJ mol–1, Cl – Cl bond energy is 242 kJ mol–1 and ΔHf for HCl is – 91 kJ mol–1.
Solution: