Gases – General Characteristics of gases
States of the matter
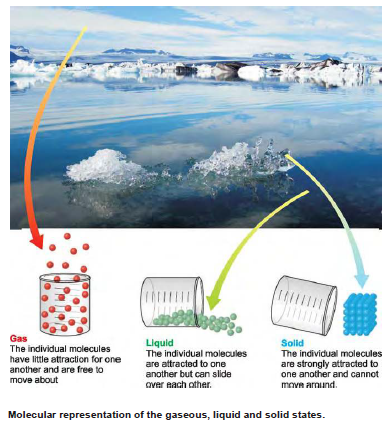
– All matter exists in three states: gases, liquids and solids.
– A gas consists of molecules separated wide apart in empty space. The molecules are free to move about throughout the container.
– A liquid has molecules touching each other. However, the intermolecular space, permits the movement of molecules throughout the liquid.
– A solid has molecules, atoms or ions arranged in a certain order in fixed positions in the crystal lattice.
– The particles in a solid are not free to move about but vibrate in their fixed positions.
– Of the three states of matter, the gaseous state is the one most studied and best understood. We shall consider it first.
General Characteristics of gases
(1) Expansibility
– Gases have limitless expansibility.
– They expand to fill the entire vessel they are placed in.
(2) Compressibility
– Gases are easily compressed by application of pressure to a movable piston fitted in the container
(3) Diffusibility
– Gases can diffuse rapidly through each other to form a homogeneous mixture.
(4) Pressure
– Gases exert pressure on the walls of the container in all directions.
(5) Effect of Heat
– When a gas, confined in a vessel is heated, its pressure increases.
– Upon heating in a vessel fitted with a piston, the volume of the gas increases.
– The above properties of gases can be easily explained by the Kinetic Molecular Theory.
Parameters of gases
– A gas sample can be described in terms of four parameters (measurable properties):
(1) the volume, V of the gas
(2) its pressure, P
(3) its temperature, T
(4) the number of moles, n, of gas in the container
(1) Volume of the gas, V
– The volume of the container is the volume of the gas sample.
– It is usually given in litre (l or L) or millilitres (ml or mL).
1 1itre(l) = 1000 ml and 1 ml = 10–3
– One millilitre is practically equal to one cubic centimetre (cc). Actually
1 litre(l) = 1000.028 cc
– The SI unit for volume is cubic metre (m3) and the smaller unit is decimeter3 (dm3).
(2) The pressure of a gas, P
– The pressure of a gas is defined as the force exerted by the impacts of its molecules per unit surface area in contact.
– The pressure of a gas sample can be measured with the help of a mercury manometer (Fig. 1) Similarly, the atmospheric pressure can be determined with a mercury barometer (Fig. 2).
– The pressure of air that can support a 760 mm Hg column at sea level, is called one atmosphere (1 atm).
– The unit of pressure, a millimetre of mercury, is also called torr. Thus,
1 atm = 760 mm Hg = 760 torr
– The SI unit of pressure is the Pascal (Pa). The relation between the atmosphere, torr and Pascal is :
1 atm = 760 torr = 1.013 × 105 Pa
– The unit of pressure (Pascal) is not in common use.
(3) Temperature, T
– The temperature of a gas may be measured in Centigrade degrees (°C) or Celsius degrees.
– The SI unit of temperature is Kelvin (K) or Absolute degree.
– The centigrade degrees can be converted to kelvins by using the equation.
– The Kelvin temperature (or absolute temperature) is always used in calculations of other parameters of gases.
– Remember that the degree sign (°) is not used with K.
(4) The Moles of a Gas Sample, n
The number of moles, n, of a sample of a gas in a container can be found by dividing the mass, m, of the sample by the molar mass, M (molecular mass).
The gas Laws
– The volume of a given sample of gas depends on the temperature and pressure applied to it.
– Any change in temperature or pressure will affect the volume of the gas.
– As a result of experimental studies from the 17th to 19th century, scientists derived the relationships among the pressure, temperature and volume of a given mass of gas.
– These relationships, which describe the general behaviour of gases, are called gas laws.
– This is called the Universal Gas Law. It is also called the Ideal Gas Law
– Ideal Gas Law as it applies to all gases which exhibit ideal behaviour i.e., obey the gas laws perfectly.
– The ideal gas law may be stated as the volume of a given amount of gas is directly proportional to the number of moles of gas, directly proportional to the temperature, and inversely proportional to the pressure.
Reference: Essentials of Physical Chemistry /Arun Bahl, B.S Bahl and G.D. Tuli / multicolour edition.