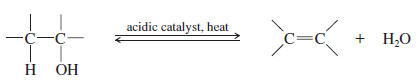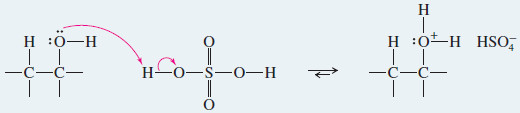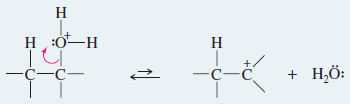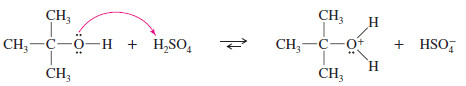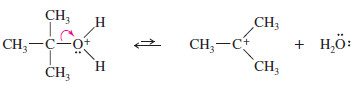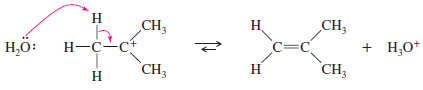Alkene Synthesis by Dehydration of Alcohols
Alkene Synthesis by Dehydration of Alcohols
– Dehydration of alcohols is a common method for making alkenes. The word dehydration literally means “removal of water.”
– Dehydration is reversible, and in most cases the equilibrium constant is not large.
– In fact, the reverse reaction (hydration) is a method for converting alkenes to alcohols.
– Dehydration can be forced to completion by removing the products from the reaction mixture as they form.
– The alkene boils at a lower temperature than the alcohol because the alcohol is hydrogen bonded.
– A carefully controlled distillation removes the alkene while leaving the alcohol in the reaction mixture Concentrated sulfuric acid and/or concentrated phosphoric acid are often used as reagents for dehydration because these acids act both as acidic catalysts and as dehydrating agents.
– Hydration of these acids is strongly exothermic.
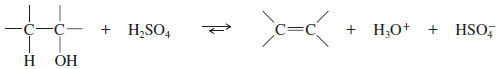
– The overall reaction (using sulfuric acid) is:
– The mechanism of dehydration resembles the E1 mechanism .
– The hydroxyl group of the alcohol is a poor leaving group (–OH), but protonation by the acidic catalyst converts it to a good leaving group (H2O).
– In the second step, loss of water from the protonated alcohol gives a carbocation.
– The carbocation is a very strong acid: Any weak base such as (H2O) or HSO4– can abstract the proton in the final step to give the alkene.
MECHANISM: Acid-Catalyzed Dehydration of an Alcohol
– Alcohol dehydrations usually involve E1 elimination of the protonated alcohol.
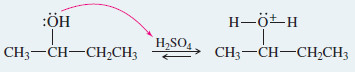
Step 1: Protonation of the hydroxyl group (fast equilibrium).
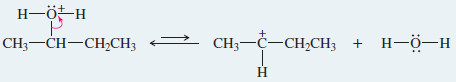
Step. 2: Ionization to a carbocation (slow; rate limiting).
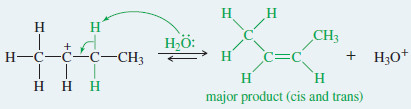
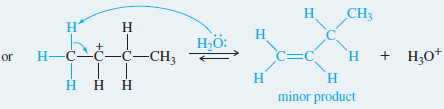
Step 3: Deprotonation to give the alkene (fast).
EXAMPLE: Acid-catalyzed dehydration of butan-2-ol
Step 1: Protonation of the hydroxyl group (fast equilibrium).
Step. 2: Ionization to a carbocation (slow; rate limiting).
Step 3: Deprotonation to give the alkene (fast).
– Like other E1 reactions, alcohol dehydration follows an order of reactivity that reflects carbocation stability: 3° alcohols react faster than 2° alcohols, and 1° alcohols are the least reactive.
– Rearrangements of the carbocation intermediates are common in alcohol dehydrations.
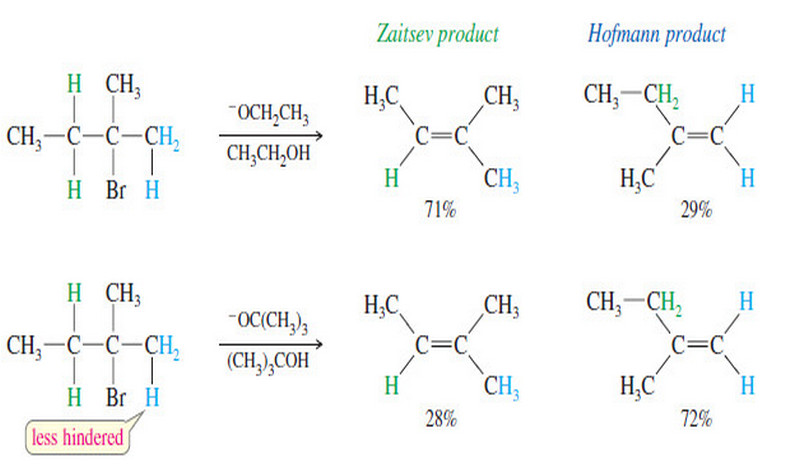
– In most cases, Zaitsev’s rule applies: The major product is usually the one with the most substituted double bond.
Solved problem:
Propose a mechanism for the sulfuric acid–catalyzed dehydration of tert-butyl alcohol.
Solution:
– The first step is protonation of the hydroxyl group, which converts it to a good leaving group.
– The second step is ionization of the protonated alcohol to give a carbocation.
– Abstraction of a proton completes the mechanism.