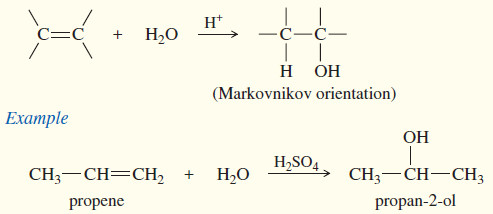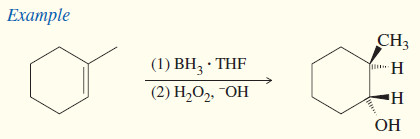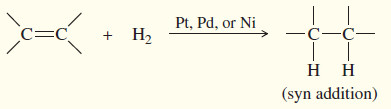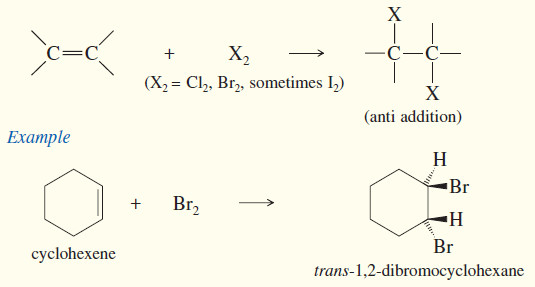Reactions of Alkenes
– In this subject we will discuss all famous reactions of alkenes.
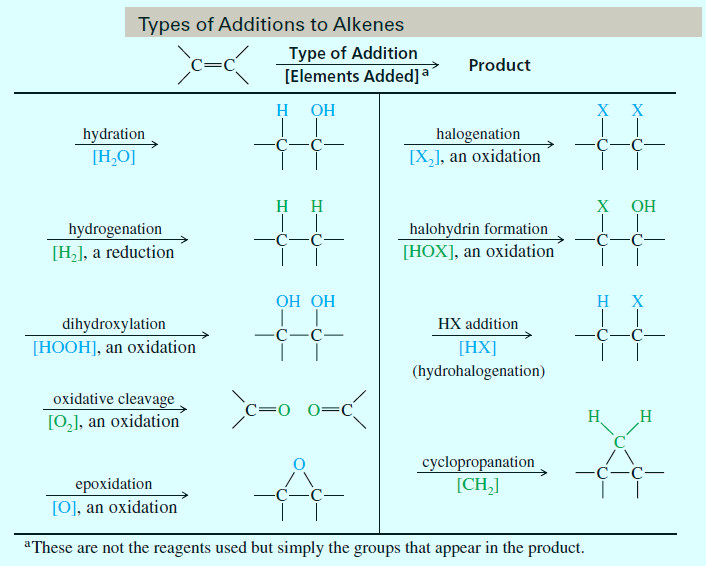
– The reactions of alkenes include five basic methods as follow:
(1) Electrophilic Additions
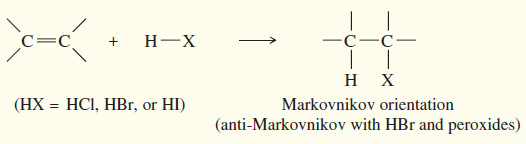
(a) Addition of hydrogen halides
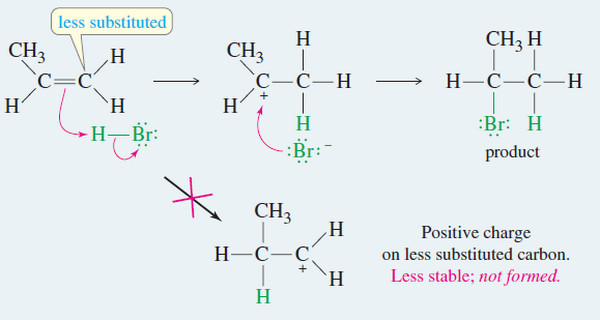
– A Russian chemist, Vladimir Markovnikov, first showed the orientation of addition of HBr to alkenes in 1869.
– Markovnikov stated: (The addition of a proton acid to the double bond of an alkene results in a product with the acid proton bonded to the carbon atom that already holds the greater number of hydrogen atoms)
– Reactions that follow this rule are said to follow Markovnikov orientation and give the Markovnikov product.
– We are often interested in adding electrophiles other than proton acids to the double bonds of alkenes.
– Markovnikov’s rule can be extended to include a wide variety of other additions, based on the addition of the electrophile in such a way as to produce the most stable carbocation.
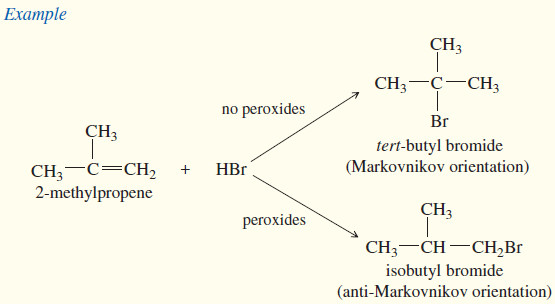
– In 1933, M. S. Kharasch and F. W. Mayo found that some additions of HBr (but not HCl or HI) to alkenes gave products that were opposite to those expected from Markovnikov’s rule.
– These anti-Markovnikov reactions were most likely when the reagents or solvents came from old supplies that had accumulated peroxides from exposure to the air.
– Peroxides give rise to free radicals that initiate the addition, causing it to occur by a radical mechanism.
(b) Acid-catalyzed hydration
An alkene may react with water in the presence of a strongly acidic catalyst to form an alcohol.
– Formally, this reaction is a hydration (the addition of water), with a hydrogen atom adding to one carbon and a hydroxyl group adding to the other.
– Hydration of alkenes are the reverse of the dehydration of alcohols.
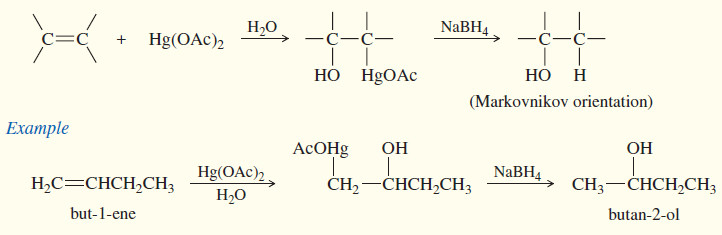
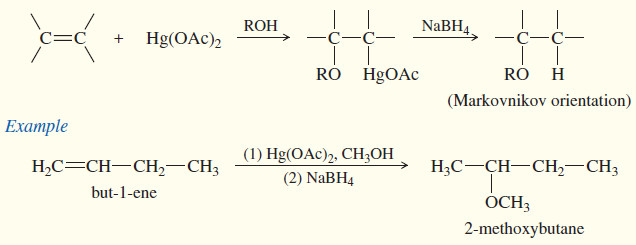
(c) Oxymercuration–demercuration
– Many alkenes do not easily undergo hydration in aqueous acid.
– Some alkenes are nearly insoluble in aqueous acid, and others undergo side reactions such as rearrangement, polymerization, or charring under these strongly acidic conditions.
– In some cases, the overall equilibrium favors the alkene rather than the alcohol.
– No amount of catalysis can cause a reaction to occur if the energetics are unfavorable.
– Oxymercuration–demercuration is another method for converting alkenes to alcohols with Markovnikov orientation.
– Oxymercuration demercuration works with many alkenes that do not easily undergo direct hydration, and it takes place under milder conditions.
– No free carbocation is formed, so there is no opportunity for rearrangements or polymerization
(d) Alkoxymercuration–demercuration
– When mercuration takes place in an alcohol solvent, the alcohol serves as a nucleophile to attack the mercurinium ion.
– The resulting product contains an alkoxy (-O-R)group.
– In effect, alkoxymercuration–demercuration converts alkenes to ethers by adding an alcohol across the double bond of the alkene
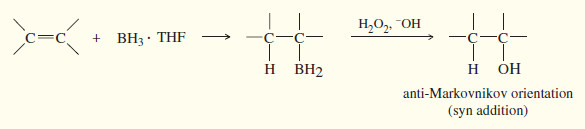
(e) Hydroboration–oxidation
The BH3 . THF reagent is the form of borane commonly used in organic reactions.
– BH3 adds to the double bond of an alkene to give an alkylborane. Basic hydrogen peroxide oxidizes the alkylborane to an alcohol.
– In effect, hydroboration–oxidation converts alkenes to alcohols by adding water across the double bond, with anti-Markovnikov orientation
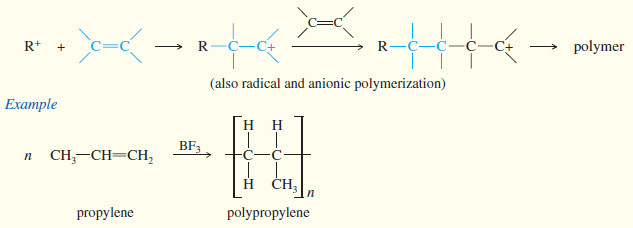
(f) Polymerization
– A polymer is a large molecule composed of many smaller repeating units (the monomers) bonded together.
– Alkenes serve as monomers for some of the most common polymers, such as polyethylene, polypropylene, polystyrene, poly(vinyl chloride), and many others.
– Alkenes polymerize to give addition polymers resulting from repeated addition reactions across their double bonds.
– Addition polymers generally form by chain-growth polymerization, the rapid addition of one molecule at a time to a growing polymer chain.
– There is generally a reactive intermediate (cation, anion, or radical) at the growing end of the chain.
– Many alkenes form addition polymers under the right conditions.
– The chain-growth mechanism involves addition of the reactive end of the growing chain across the double bond of the alkene monomer.
– Depending on the conditions and the structure of the monomer, the reactive intermediates may be carbocations, free radicals, or carbanions
(2) Reduction: Catalytic Hydrogenation
– Hydrogenation of an alkene is formally a reduction, with H2 adding across the double bond to give an alkane.
– The process usually requires a catalyst containing Pt, Pd, or Ni.
– For most alkenes, hydrogenation takes place at room temperature, using hydrogen gas at atmospheric pressure.
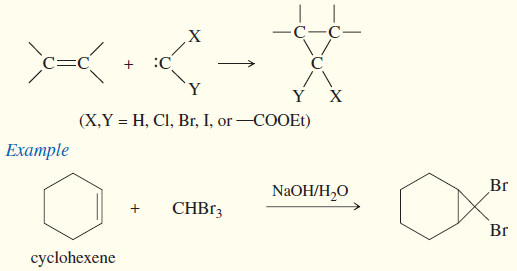
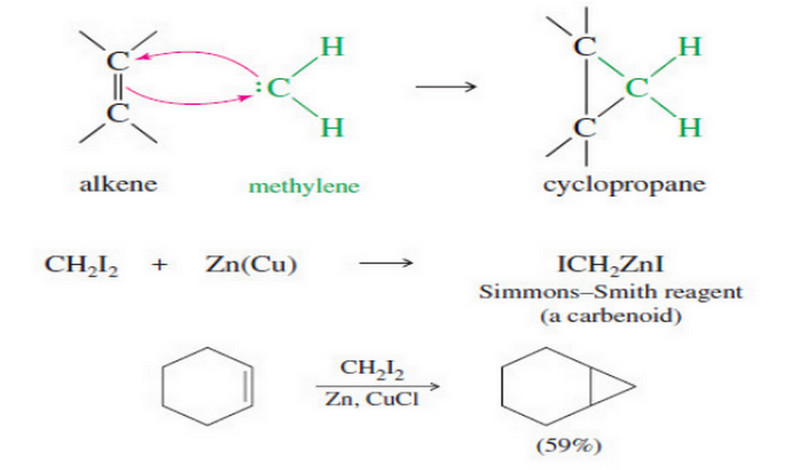
(3) Addition of Carbenes: Cyclopropanation
– Carbenes are also formed by reactions of halogenated compounds with bases.
– If a carbon atom has bonds to at least one hydrogen and to enough halogen atoms to make the hydrogen slightly acidic, it may be possible to form a carbene.
– For example, bromoform (CHBr3) reacts with a 50% aqueous solution of potassium hydroxide to form dibromocarbene.
– This dehydrohalogenation is called an alpha elimination (α elimination) because the hydrogen and the halogen are lost from the same carbon atom.
– The more common dehydrohalogenations (to form alkenes) are called beta eliminations (β elimination) because the hydrogen and the halogen are lost from adjacent carbon atoms.
(4) Oxidative Additions
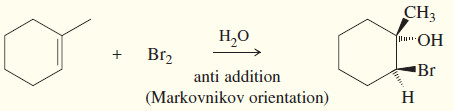
(a) Addition of halogens
– Addition of Halogens to Alkenes gives vicinal dihalides.
(b) Halohydrin formation
– A halohydrin is an alcohol with a halogen on the adjacent carbon atom.
– In the presence of water, halogens add to alkenes to form halohydrins.
– The electrophilic halogen adds to the alkene to give a halonium ion, which is also electrophilic.
– Water acts as a nucleophile to open the halonium ion and form the halohydrin.
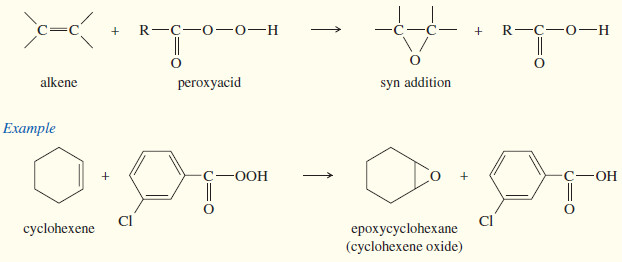
(c) Epoxidation
– Some of the most important reactions of alkenes involve oxidation.
– When we speak of oxidation, we usually mean reactions that form carbon–oxygen bonds. (Halogens are oxidizing agents, and the addition of a halogen molecule across a double bond is formally an oxidation as well.)
– Oxidations are particularly important because many common functional groups contain oxygen, and alkene oxidations are some of the best methods for introducing oxygen into organic molecules.
– We will consider methods for epoxidation, dihydroxylation, and oxidative cleavage of alkene double bonds.
– An epoxide is a three-membered cyclic ether, also called an oxirane.
– Epoxides are valuable synthetic intermediates used for converting alkenes to a variety of other functional groups.
– An alkene is converted to an epoxide by a peroxyacid, a carboxylic acid that has an extra oxygen atom in a -O-O- (peroxy) linkage.
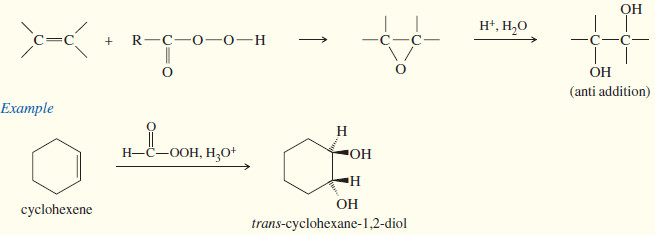
(d) Anti dihydroxylation
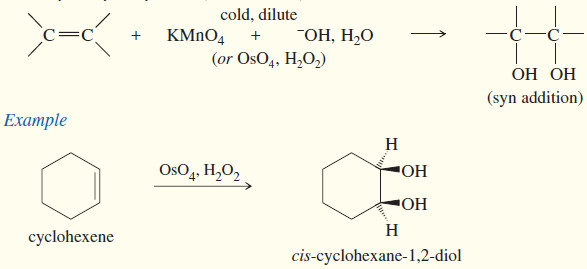
(e) Syn dihydroxylation
– Converting an alkene to a glycol requires adding a hydroxyl group to each end of the double bond. This addition is called Dihydroxylation of Alkenes or dihydroxylation (or hydroxylation) of the double bond.
– We have seen that epoxidation of an alkene, followed by acidic hydrolysis, gives anti dihydroxylation of the double bond.
– Reagents are also available for the dihydroxylation of alkenes with syn stereochemistry.
– The two most common reagents for this purpose are osmium tetroxide and potassium permanganate.
(5) Oxidative Cleavage of Alkenes
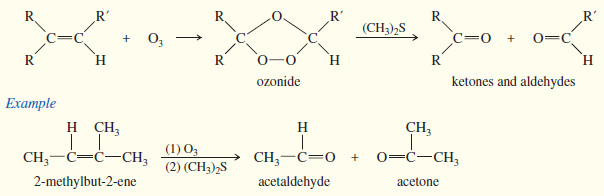
(a) Ozonolysis
ozone cleaves double bonds to give ketones and aldehydes. However, ozonolysis is milder, and both ketones and aldehydes can be recovered without further oxidation.
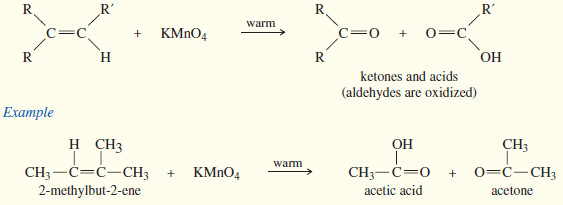
(b) Potassium permanganate
– In a potassium permanganate dihydroxylation, if the solution is warm or acidic or too concentrated, oxidative cleavage of the glycol may occur.
– In effect, the double bond is cleaved to two carbonyl groups.
– The products are initially ketones and aldehydes, but aldehydes are oxidized to carboxylic acids under these strong oxidizing conditions.
– If the molecule contains a terminal =CH2 group, that group is oxidized all the way to CO2 and water.
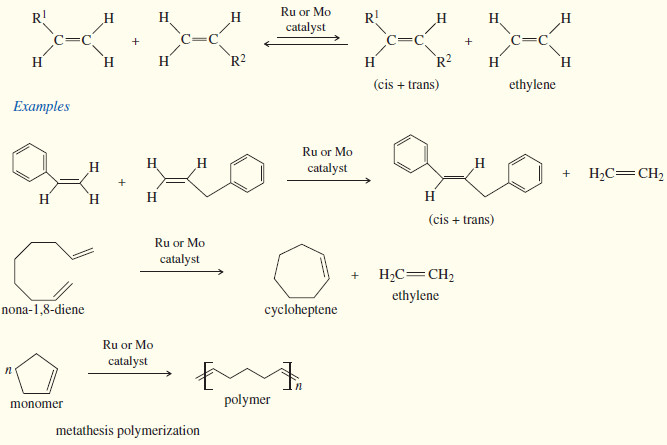
(6) Olefin (Alkene) Metathesis
– The double bond is the strongest bond in an alkene, yet it is also the most reactive bond.
– Imagine how useful it would be if we could break molecules at their double bonds and reassemble them as we please. That is the goal of olefin metathesis.
– We can think of an alkene as two alkylidene groups (=CHR) held together by the double bond, and mentally divide it up just like we divide the molecule when we go to name it as E or Z .
– Olefin metathesis is any reaction that trades and interchanges these alkylidene groups.
– The word metathesis comes from the Greek words meta (change) and thesis (position), meaning that the alkylidene groups change their positions in the products.