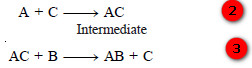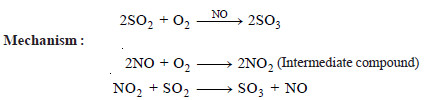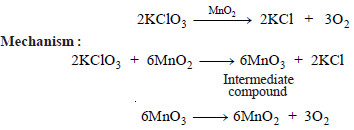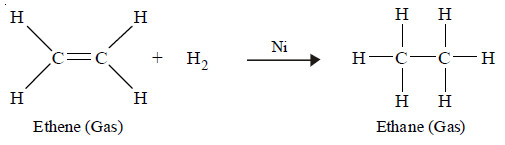Theories of catalysis
Theories of Catalysis
– There are two main theories of catalysis:
(1) Intermediate Compound Formation theory.
(2) The Adsorption theory.
– In general, the Intermediate Compound Formation theory applies to homogeneous catalytic reactions and the Adsorption theory applies to heterogeneous catalytic reactions.
The Intermediate Compound Formation Theory
– The Intermediate Compound Formation Theory is the first theory of theories of catalysis.
– As already discussed a catalyst functions by providing a new pathway of lower activation energy.
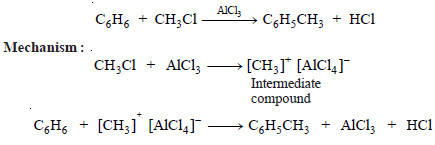
– In homogeneous catalysis, it does so by forming an intermediate compound with one of the reactants.
– The highly reactive intermediate compound then reacts with the second reactant to yield the product, releasing the catalyst.
– Let us illustrate it by taking the general reaction:
where C acts as catalyst.
– The reaction proceeds through the reactions :
– The activation energies of the reactions (2) and (3) are lower than that of the reaction (1) Hence the involvement of the catalyst in the formation of the intermediate compound and its subsequent decomposition, accelerates the rate of the reaction (1) which was originally very slow.
Examples
Example (1): Catalytic oxidation of sulphur dioxide (SO2) in the presence of nitric oxide (NO) as catalyst. (Chamber Process of Sulphuric acid).
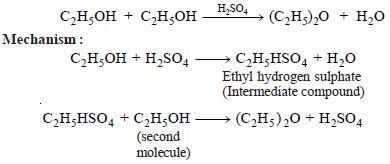
Example (2): Preparation of diethyl ether, (C2H5)2O, from ethanol (C2H5OH) using sulphuric acid as catalyst.
Example(3): Thermal decomposition of potassium chlorate (KClO3) in the presence of manganese dioxide (MnO2).
Example (4): Formation of methylbenzene, C6H5CH3 by reaction between benzene, C6H6, and methyl chloride, CH3Cl, using aluminium chloride AlCl3, as catalyst (Friedel-Crafts reaction)
– It may be noted that the actual isolation of intermediate compounds which would prove their existence is very difficult. As already stated, by their very nature they are unstable.
– In general, the intermediate compounds suggested as being formed are usually plausible rather that proved.
The Adsorption Theory
– The Adsorption Theory is the second theory of theories of catalysis.
– This theory explains the mechanism of a reaction between two gases catalysed by a solid (Heterogeneous or Contact Catalysis).
– Here the catalyst functions by adsorption of the reacting molecules on its surface.
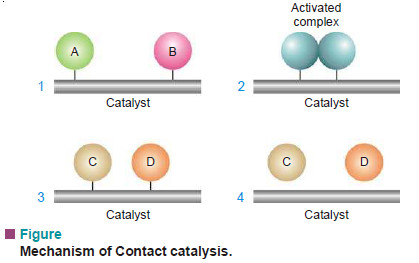
– Generally speaking, four steps can be put forward for heterogeneous catalysis.
– For example, if the reaction is :
Step (1): Adsorption of Reactant molecules
– The reactant molecules A and B strike the catalyst surface.
– They are held up at the surface by weak van der Waals forces (Physical adsorption) or by partial chemical bonds (Chemisorption).
Step (2): Formation of Activated complex
– The particles of the reactants adjacent to one another join to form an intermediate complex (A – B).
– The activated complex is unstable. It has only fleeting existence.
Step (3): Decomposition of Activated complex
– The activated complex breaks to form the products C and D.
– The separated particles of the products hold to the catalyst surface by partial chemical bonds.
Step (4): Desorption of Products
– The particles of the products are desorbed or released from the surface.
– They are stable and can lead an independent existence.
– The mechanism of contact catalysis may vary in details, depending on the nature of the reactants.
Hydrogenation of ethene (ethylene) in presence of nickel
– Ethene adds hydrogen in the presence of nickel as a catalyst to yield ethane.
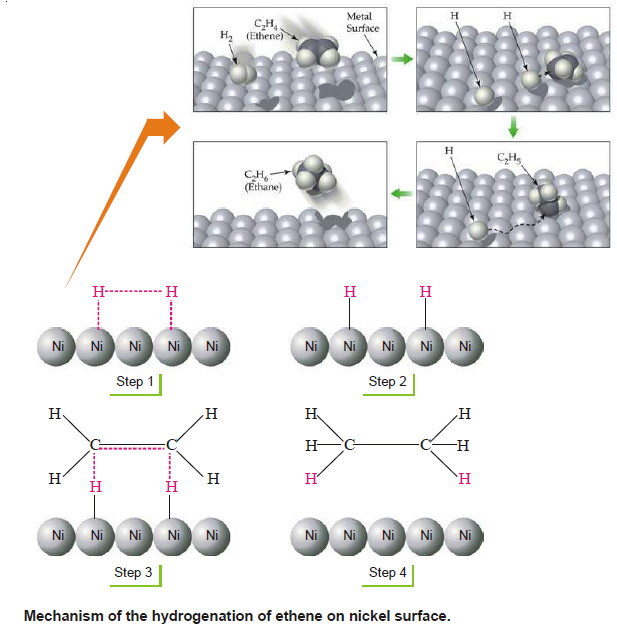
– The catalyst operates by the following steps:
Step (1): Adsorption of Hydrogen molecules
– Hydrogen molecules are adsorbed on the nickel surface due to the residual valence bonds of the nickel atoms.
Step (2): H–H Bonds are broken
– The H–H bond is smaller (0.74Å) than Ni–Ni bond. Therefore, the H–H bond of the adsorbed hydrogen molecule is stretched and weakened.
– The weakened bond breaks, separating the hydrogen atoms.
– The separated hydrogen atoms are held to the nickel surface by chemical bonds.
Step (3): Formation of the Activated complex
– The chemisorbed hydrogen atoms then attach to ethene molecule by partial chemical bonds.
– The unstable activated complex is thus formed.
Step (4): Decomposition of the Activated complex and desorption of ethane molecules
– The unstable activated complex decomposes to release ethane molecules.
– The freed catalyst surface is again available for further action.
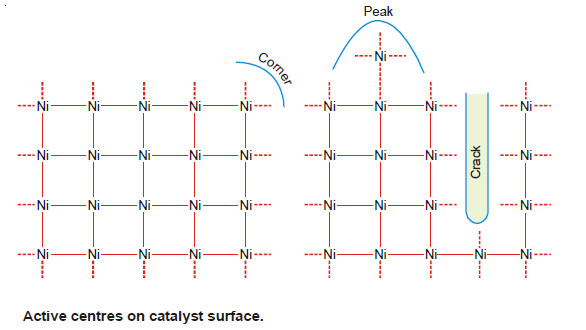
Active Centres on Catalyst Surface
– Just like surface tension, the catalyst has unbalanced chemical bonds on it.
– The reactant gaseous molecules are adsorbed on the surface by these free bonds. This accelerates the rate of the reaction.
– The distribution of free bonds on the catalyst surface is not uniform. These are crowded at the ‘peaks’, ‘cracks’ and ‘corners’ of the catalyst.
– The catalytic activity due to adsorption of reacting molecules is maximum at these spots. These are, therefore, referred to as the active centres.
– The active centres increase the rate of reaction not only by increasing the concentration of the reactants but they also activate the molecule adsorbed at two such centres by stretching it.
The Adsorption Theory Explains Catalytic Activity
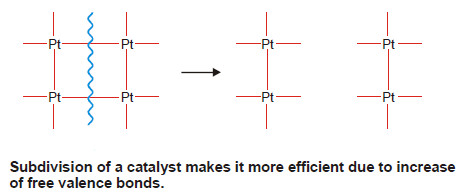
(1) Metals in a state of fine subdivision or colloidal form are rich in free valence bonds and hence they are more efficient catalysts than the metal in lumps.
(2) Catalytic poisoning occurs because the so-called poison blocks the free valence bonds on its surface by preferential adsorption or by chemical combination.
(3) A promoter increases the valence bonds on the catalyst surface by changing the crystal lattice and thereby increasing the active centres.