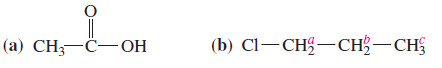Chemical Shift in NMR Spectroscopy
– In this topic, we will discuss the Chemical Shift in 1H NMR Spectroscopy.
What is Chemical Shift?
– The variations in the positions of NMR absorptions, arising from electronic shielding and deshielding, are called chemical shifts.
– Chemical shift is The difference (in parts per million) between the resonance frequency of the proton being observed and that of tetramethylsilane (TMS).
Measurement of Chemical Shift
– In practice, it is difficult to measure the absolute field where a proton absorbs with enough accuracy to distinguish individual protons, because the signals often differ by only a few thousandths of a gauss at an applied field of 70,459 gauss.
– A more accurate method for expressing chemical shifts is to determine the value relative to a reference compound added to the sample.
– The difference in the magnetic field strength between the resonances of the sample protons and the reference protons can be measured very accurately.
– The most common NMR reference compound is tetramethylsilane abbreviated TMS.
– Because silicon is less electronegative than carbon, the methyl groups of TMS are relatively electron-rich, and their protons are well shielded.
– They absorb at a higher field strength than most hydrogens bonded to carbon or other elements, so most NMR signals appear downfield (to the left, deshielded) of the TMS signal.
– All 12 protons in TMS absorb at exactly the same applied magnetic field, giving one strong absorption.
– A small amount of TMS is added to the sample, and the instrument measures the difference in magnetic field between where the protons in the sample absorb and where those in TMS absorb.
– For each type of proton, the distance downfield of TMS is the chemical shift of those protons.
– Newer spectrometers operate at a constant magnetic field, and they measure the chemical shift as a frequency difference between the resonances of the protons in the sample and those in TMS.
– Remember that frequency units (v ) and magnetic field units (B0) are always proportional in NMR, with
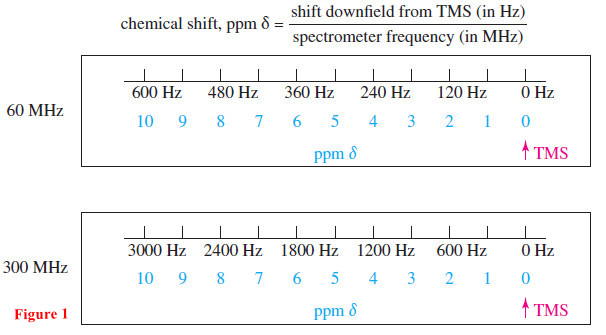
– Chemical shifts are measured in parts per million (ppm), a dimensionless fraction of either the total applied field or the total radio frequency.
– By custom, the difference (the chemical shift) between the NMR signal of a proton and that of TMS is shown on the horizontal axis of the NMR spectrum calibrated in frequency units (hertz or Hz).
– A chemical shift in parts per million can be calculated by dividing the shift measured in hertz by the spectrometer frequency measured in millions of hertz (megahertz or MHz).
– In a 300-MHz (300,000,000 Hz) spectrum, for example, 1 ppm = 300 Hz.
– The chemical shift (in ppm) of a given proton is the same regardless of the operating field and frequency of the spectrometer.
– The use of dimensionless chemical shifts to locate absorptions standardizes the values for all NMR spectrometers.
– The most common scale of chemical shifts is the δ (delta) scale, which we will use (Figure 1).
– The signal from tetramethylsilane (TMS) is defined as 0.00 ppm on the δ scale.
– Most protons are more deshielded than TMS, so the δ scale increases toward the left of the spectrum.
– The spectrum is calibrated in both frequency and ppm δ.
Solved Problem (1)
A 300-MHz spectrometer records a proton that absorbs at a frequency 2130 Hz downfield (deshielded) from TMS.
(a) Determine its chemical shift.
(b) Predict this proton’s chemical shift at 60 MHz. In a 60-MHz spectrometer, how far downfield (in hertz) from TMS would this proton absorb?
Solution:
(a) The chemical shift is the fraction:
(b) The chemical shift is unchanged at 60 MHz: δ 7.10.The frequency shift is
60.00 MHz × (7.10 × 10-6) = 426 Hz
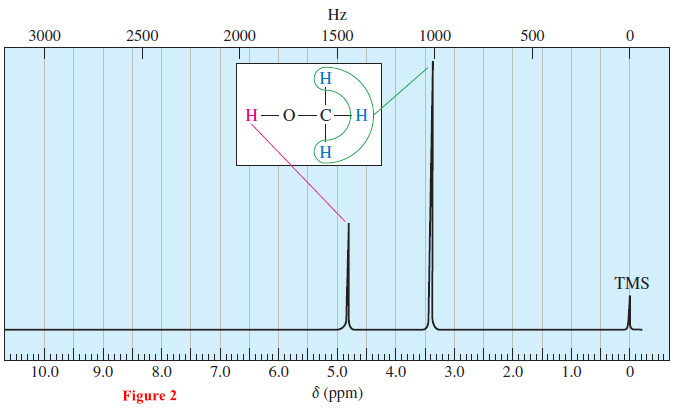
– The 300-MHz NMR spectrum of methanol (Figure 2) shows the two signals from methanol together with the TMS reference peak at δ 0.0.
– The methyl protons absorb 1025 Hz downfield from TMS. Their chemical shift is 3.4 ppm, so we say that the methyl protons absorb at δ 3.4.
– The hydroxyl proton absorbs farther downfield, at a position around 1450 Hz from TMS. Its chemical shift is δ 4.8.
– Both the hydroxyl proton and the methyl protons of methanol show the deshielding effects of the electronegative oxygen atom.
– The chemical shift of a methyl group in an alkane is about δ 0.9.Therefore, the methanol oxygen deshields the methyl protons by an additional 2.5 ppm.
– Other electronegative atoms produce similar deshielding effects.
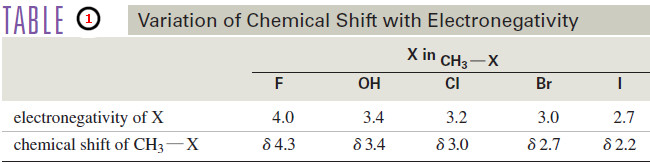
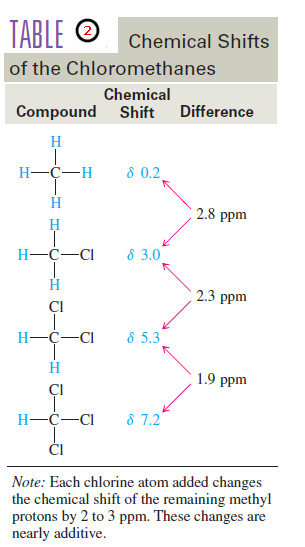
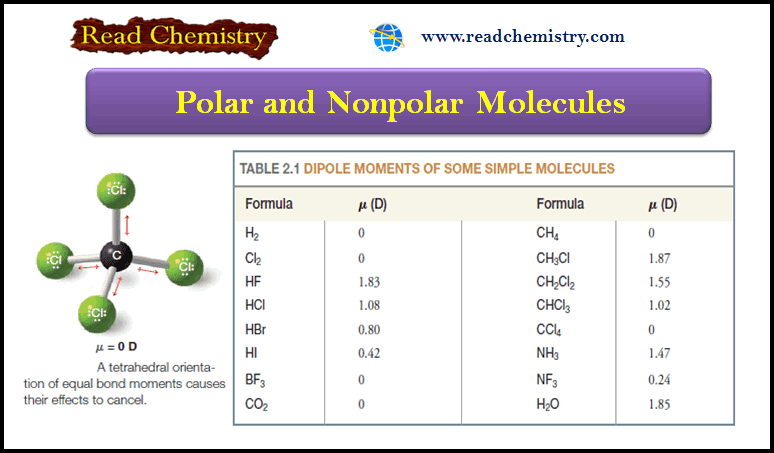
– Table (1) compares the chemical shifts of methanol with those of the methyl halides.
– Notice that the chemical shift of the methyl protons depends on the electronegativity of the substituent, with more electronegative substituents deshielding more and giving larger chemical shifts.
– The effect of an electronegative group on the chemical shift also depends on its distance from the protons.
– In methanol, the hydroxyl proton is separated from oxygen by one bond, and its chemical shift is δ 4.8.
The methyl protons are separated from oxygen by two bonds, and their chemical shift is δ 4.8.
– In general, the effect of an electron-withdrawing substituent decreases with increasing distance, and the effects are usually negligible on protons that are separated from the electronegative group by four or more bonds.
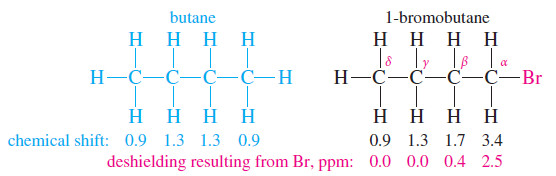
– This decreasing effect can be seen by comparing the chemical shifts of the various protons in 1-bromobutane with those in butane.
– The deshielding effect of an electronegative substituent drops off rapidly with distance. In 1-bromobutane, protons on the are α carbon deshielded by about 2.5 ppm, and the β protons are deshielded by about 0.4 ppm.
– Protons that are more distant than the β protons are deshielded by a negligible amount.
– If more than one electron-withdrawing group is present, the deshielding effects
are nearly (but not quite) additive.
– In the chloromethanes (Table 2), the addition of the first chlorine atom causes a shift to δ 3.0, the second chlorine shifts the absorption further to δ 5.3, and the third chlorine moves he chemical shift δ 7.2 to for chloroform.
– The chemical shift difference is about 2 to 3 ppm each time another chlorine atom is added, but each additional chlorine moves the peak slightly less than the previous one did.
Characteristic Values of Chemical Shifts
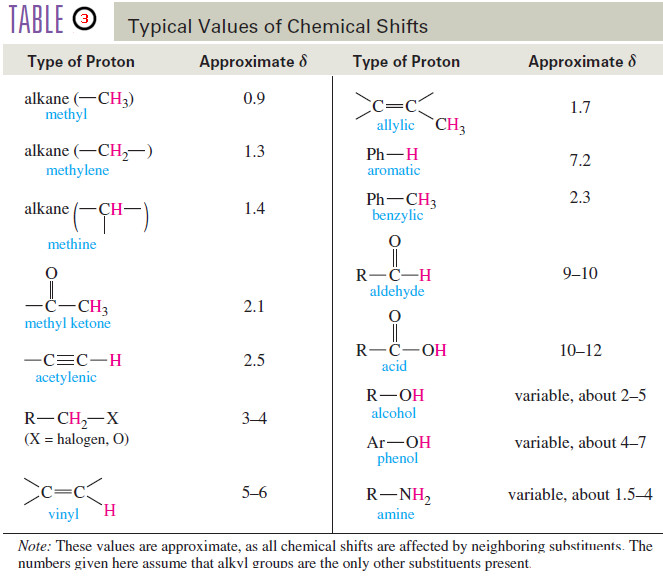
– Since the chemical shift of a proton is determined by its environment, we can construct a table of approximate chemical shifts for many types of compounds.
– Let’s begin with a short table of representative chemical shifts (Table 3) and consider the reasons for some of the more interesting and unusual values.
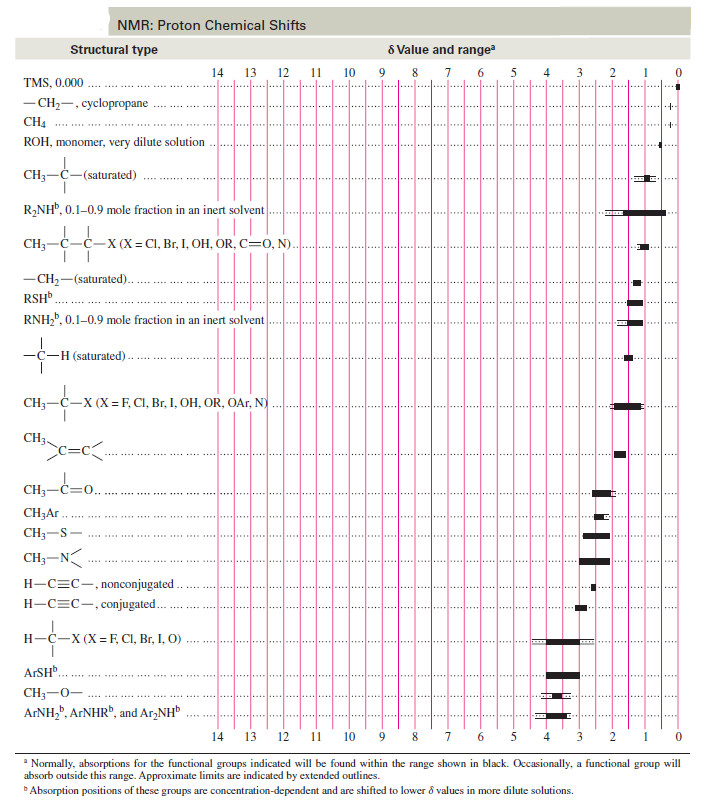
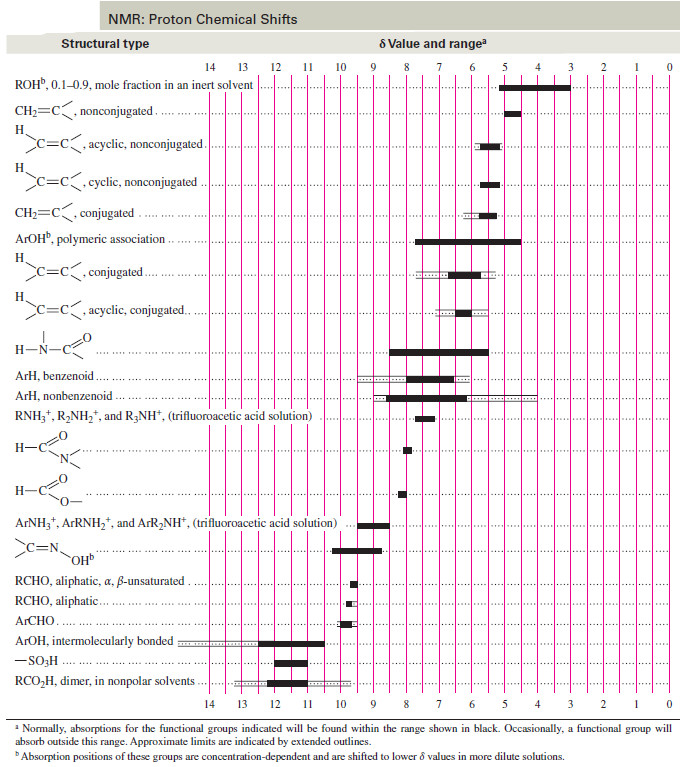
– A comprehensive table of chemical shifts appears as follow:
Solved Problem (2)
Using Table 3, predict the chemical shifts of the protons in the following compounds.
Solution:
(a) The methyl group in acetic acid is next to a carbonyl group; Table 3 predicts a chemical shift of about δ 2.1.(The experimental value is δ 2.10) The acid proton(-COOH) should absorb between δ 10 and δ 12.(The experimental value is δ 11.4, variable.)
(b) Protons a are on the carbon atom bearing the chlorine, and they absorb between δ 3 and δ 4. (experimental: δ 3.7). Protons b are one carbon removed, and they are predicted to absorb aboutδ 1.7, like the β protons in 1-bromobutane (experimental: δ 1.8 ). The methyl protons c will be nearly unaffected, absorbing around δ 0.9 ppm (experimental: δ 1.0).
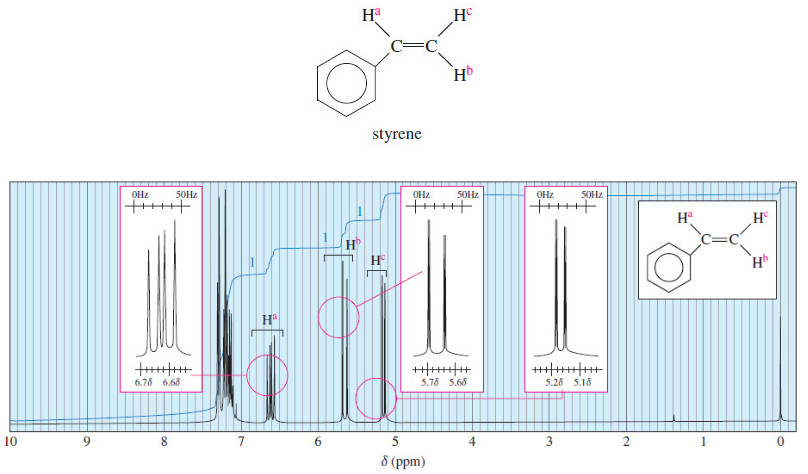
(c) Methyl protons a are expected to absorb around δ 0.9 (experimental: δ 1.0). The vinyl protons b and c are expected to absorb between δ 5 and δ 6 (experimental δ 5.8 for b and d4.9 for c).
Vinyl and Aromatic Protons
– Table 3 shows that double bonds and aromatic rings produce large deshielding effects on their vinyl and aromatic protons.
– These deshielding effects result from the same type of circulation of electrons that normally shields nuclei from the magnetic field.
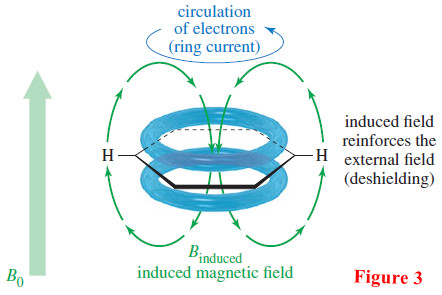
– In benzene and its derivatives, the aromatic ring of pi bonding electrons acts as a conductor, and the external magnetic field induces a ring current (Figure 3).
– At the center of the ring, the induced field acts to oppose the external field.
– These induced field lines curve around, however, and on the edge of the ring the induced field adds to the external field.
– As a result, the aromatic protons are strongly deshielded, resulting in a large chemical shift.
– Benzene absorbs at δ 7.2, and most aromatic protons absorb in the range δ 7 of δ 8.
– The benzene molecule is not always lined up in the position shown in Figure 3.
– Because benzene is constantly tumbling in the solution, the chemical shift observed for its protons is an average of all the possible orientations.
– If we could hold a benzene molecule in the position shown in Figure 3, its protons would absorb at a field even lower than Other orientations, such as the one with the benzene ring edge-on to the magnetic field, would be less deshielded and would absorb at a higher field. It is the average of all these orientations that is observed by the resonance at δ 7.2.
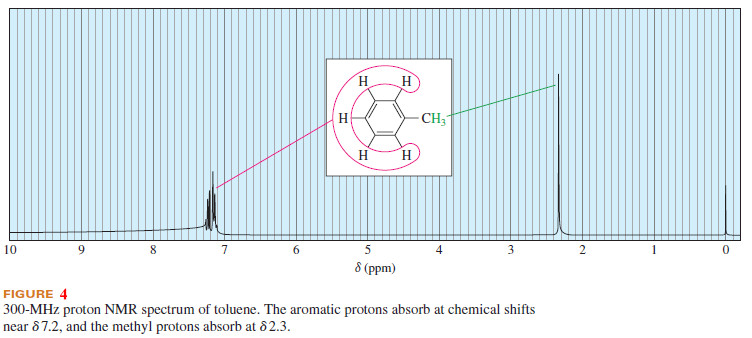
– Figure 4 shows the NMR spectrum of toluene (methylbenzene). The aromatic protons absorb around δ 7.2.
– The methyl protons are deshielded by a smaller amount, absorbing at δ 2.3.
– The pi electrons of an alkene deshield the vinyl protons in the same way that an aromatic ring of electrons deshields the aromatic protons.
– The effect is not as large in the alkene, however, because there is not such a large, effective ring of electrons as there is in benzene.
– Once again, the motion of the pi electrons generates an induced magnetic field that opposes the applied field at the middle of the double bond.
– The vinyl protons are on the periphery of this field, however, where the induced field bends around and reinforces the external field (Figure 5).
– As a result of this deshielding effect, most vinyl protons absorb in the range of δ 5 to δ 6.
Acetylenic Hydrogens
– Since the pi bond of an alkene deshields the vinyl protons, we might expect an acetylenic hydrogen (-C≡C-H) to be even more deshielded by the two pi bonds of the triple bond.
– The opposite is true: Acetylenic hydrogens absorb around δ 2.5, compared with δ 5 to δ 6 for vinyl protons.
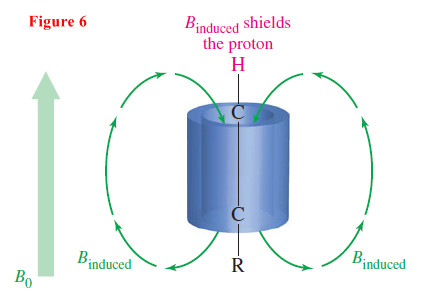
– Figure 6 shows that the triple bond has a cylinder of electron density surrounding the sigma bond.
– As the molecules tumble in solution, in some orientations this cylinder of electrons can circulate to produce an induced magnetic field.
– The acetylenic proton lies along the axis of this induced field, which is a shielded region.
– When this shielded orientation is averaged with all other possible (mostly deshielded) orientations, the result is a resonance around δ 2.5.
Aldehyde Protons
– Aldehyde protons (-CHO) absorb at even lower fields than vinyl protons and aromatic protons: between δ 9 and δ 10.
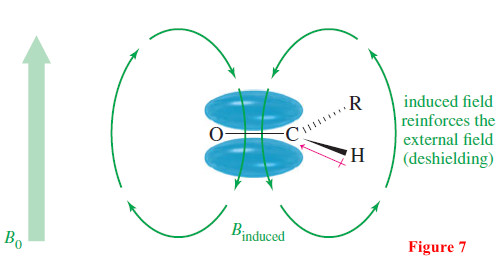
– Figure 7 shows that the aldehyde proton is deshielded both by the circulation of the electrons in the double bond and by the inductive electron-withdrawing effect of the carbonyl oxygen atom.
Hydrogen-Bonded Protons
– The chemical shifts of O-H protons in alcohols and N-H protons in amines depend on the concentration.
– In concentrated solutions, these protons are deshielded by hydrogen bonding, and they absorb at a relatively low field: δ 3.5 about for an amine N-H and about δ 4.5 for an alcohol O-H.
– When the alcohol or amine is diluted with a non-hydrogen-bonding solvent such as CCl4, hydrogen bonding becomes less important.
– In dilute solutions, these signals are observed around δ 2.
– Hydrogen bonding and the proton exchange that accompanies it may contribute to a broadening of the peak from an O-H or N-H proton.
– A broad peak appears because protons exchange from one molecule to another during the NMR resonance .
– The protons pass through a variety of environments during this exchange, absorbing over a wider range of frequencies and field strengths.
Carboxylic Acid Protons
– Because carboxylic acid protons are bonded to an oxygen next to a carbonyl group, they have considerable positive character.
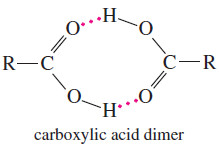
– They are strongly deshielded and absorb at chemical shifts greater than δ 10. Carboxylic acids frequently exist as hydrogen-bonded dimers (shown at left), with moderate rates of proton exchange that broaden the absorption of the acid proton.
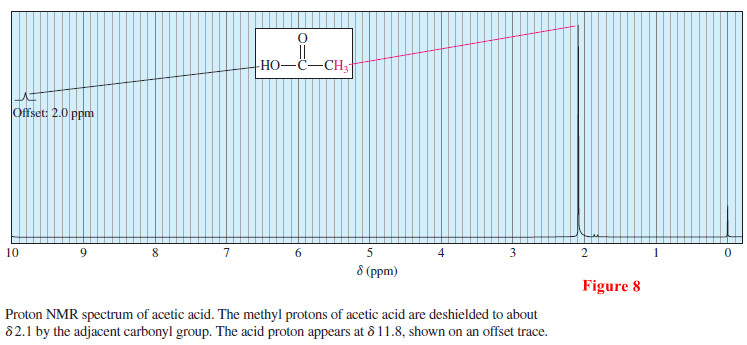
– The proton NMR spectrum of acetic acid is shown in Figure 8.
As we expect, the methyl group next to the carbonyl absorbs at a chemical shift of δ 2.1.
– The acid proton signal appears at a chemical shift that is not scanned in the usual range of the NMR spectrum.
– It is seen in a second trace with a 2.0 ppm offset, meaning that this trace corresponds to frequencies with chemical shifts 2.0 ppm larger than shown on the trace.
– The acid proton appears around δ 11.8: the sum of δ 9.8 read from the trace, plus the δ 2.0 offset.
References:
- Organic chemistry / L.G. Wade, Jr / 8th ed, 2013 / Pearson Education, Inc. USA.
- Fundamental of Organic Chemistry / John McMurry, Cornell University/ 8th ed, 2016 / Cengage Learningm, Inc. USA.
- Organic Chemistry / T.W. Graham Solomons, Craig B. Fryhle , Scott A. Snyder / 11 ed, 2014/ John Wiley & Sons, Inc. USA.