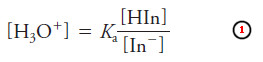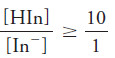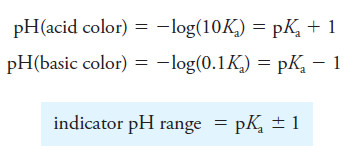Indicators and Solutions for acid-base titration
– In this topic, we will discuss the Indicators and Solutions for acid-base titration.
Indicators and Solutions for acid-base titration
– Like all titrations, neutralization titrations depend on a chemical reaction of the analyte with a standard reagent.
– There are several different types of acid/base titrations.
– One of the most common is the titration of a strong acid, such as hydrochloric or sulfuric acid, with a strong base, such as sodium hydroxide.
– Another common type is the titration of a weak acid, such as acetic or lactic acid, with a strong base.
– Weak bases, such as sodium cyanide or sodium salicylate, can also be titrated with strong acids.
– In all titrations, we must have a method of determining the point of chemical equivalence.
– Typically, a chemical indicator or an instrumental method is used to locate the end point, which we hope is very close to the equivalence point.
– Our discussion focuses on the types of standard solutions and the chemical indicators that are used for neutralization titrations.
Standard Solutions
– The standard solutions used in neutralization titrations are strong acids or strong bases because these substances react more completely with an analyte than do weak acids and bases, and as a result, they produce sharper end points.
– Standard solutions of acids are prepared by diluting concentrated hydrochloric, perchloric, or sulfuric acid.
– Nitric acid is seldom used because its oxidizing properties offer the potential for undesirable side reactions.
– Hot concentrated perchloric and sulfuric acids are potent oxidizing agents and are very hazardous.
– Fortunately, cold dilute solutions of these reagents are safe to use in the analytical laboratory without any special precautions other than eye protection.
– Standard solutions of bases are usually prepared from solid sodium, potassium, and occasionally barium hydroxides.
– Again, always use eye protection when handling dilute solutions of these reagents.
Acid/Base Indicators
– Many naturally occurring and synthetic compounds exhibit colors that depend on the pH of the solutions in which they are dissolved.
– Some of these substances, which have been used for centuries to indicate the acidity or alkalinity of water, are still applied today as acid/base indicators.
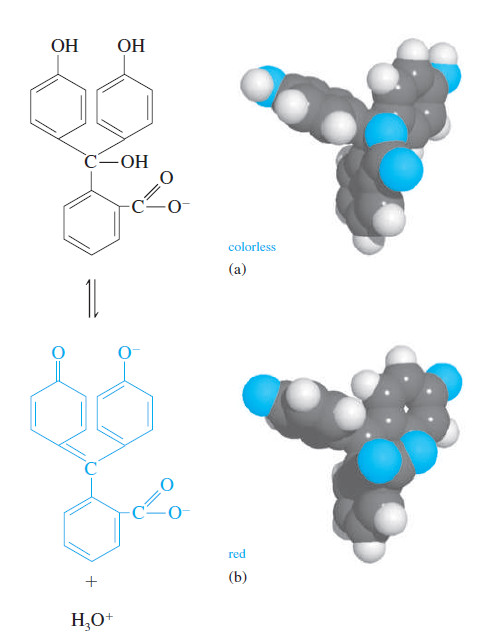
– An acid/base indicator is a weak organic acid or a weak organic base whose undissociated form differs in color from its conjugate base or its conjugate acid form.
– For example, the behavior of an acid-type indicator, HIn, is described by the equilibrium:
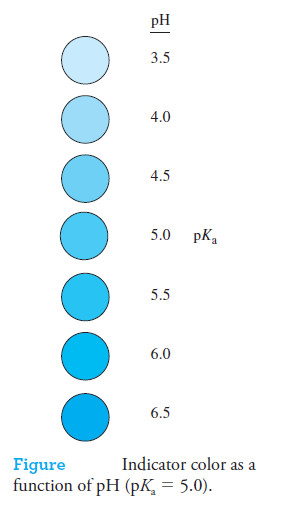
In this reaction, internal structural changes accompany dissociation and cause the color
change (for example, see Figure).
– The equilibrium for a base-type indicator, In, is:
– In the paragraphs that follow, we focus on the behavior of acid-type indicators.
– The principles can be easily extended to base-type indicators as well.
The equilibrium-constant expression for the dissociation of an acid-type indicator takes the form:
Rearranging leads to:
– We see then that the hydronium ion is proportional to the ratio of the concentration of the acid form to the concentration of the base form of the indicator, which in turn controls the color of the solution.
– The human eye is not very sensitive to color differences in a solution containing a mixture of HIn and In–, particularly when the ratio [HIn]/[In–] is greater than about 10 or smaller than about 0.1.
– Because of this restriction, the color change detected by an average observer occurs within a limited range of concentration ratios from about 10 to about 0.1.
– At greater or smaller ratios, the color appears essentially constant to the eye and is independent of the ratio.
– As a result, we can write that the average indicator, HIn, exhibits its pure acid color when
and its base color when:
– The color appears to be intermediate for ratios between these two values. These ratios vary considerably from indicator to indicator.
– Furthermore, people differ significantly in their ability to distinguish between colors.
– If we substitute the two concentration ratios into Equation (1), the range of hydronium ion concentrations needed for the indicator to change color can be estimated.
For full acid color,
and for the full base color,
– To obtain the indicator pH range, we take the negative logarithms of the two expressions:
– This expression shows that an indicator with an acid dissociation constant of 1 × 10-5 (pKa = 5) typically shows a complete color change when the pH of the solution in which it is dissolved changes from 4 to 6 (see the following Figure).
– We can derive a similar relationship for a basic-type indicator.
Titration Errors with Acid/Base Indicators
– We find two types of titration errors in acid/base titrations.
– The first is a determinate error that occurs when the pH at which the indicator changes color differs from the pH at the equivalence point.
– This type of error can usually be minimized by choosing the indicator carefully or by making a blank correction.
– The second type is an indeterminate error that originates from the limited ability of the human eye to distinguish reproducibly the intermediate color of the indicator.
– The magnitude of this error depends on the change in pH per milliliter of reagent at the equivalence point, on the concentration of the indicator, and on the sensitivity of the eye to the two indicator colors.
– On average, the visual uncertainty with an acid/base indicator is in the range of ±0.5 to ±1 pH unit.
– This uncertainty can often be decreased to as little as ±0.1 pH unit by matching the color of the solution being titrated with that of a reference standard containing a similar amount of indicator at the appropriate pH.
– These uncertainties are approximations that vary considerably from indicator to indicator as well as from person to person.
Variables That Influence the Behavior of Indicators
– The pH interval over which a given indicator exhibits a color change is influenced by temperature, by the ionic strength of the medium, and by the presence of organic solvents and colloidal particles.
– Some of these effects, particularly the last two, can cause the transition range to shift by one or more pH units.
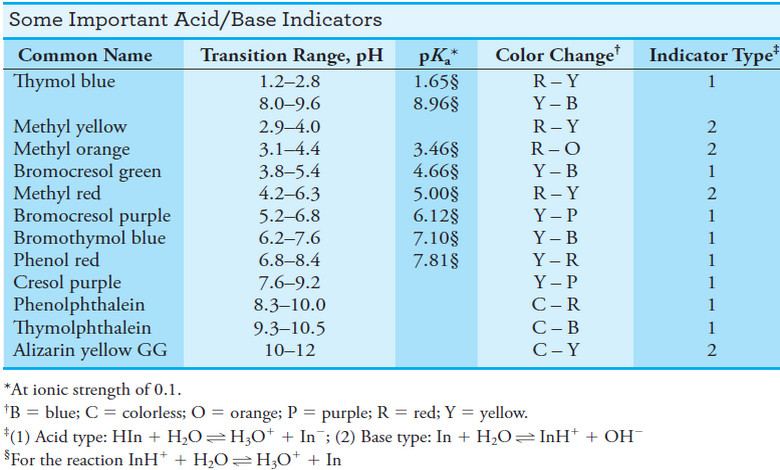
The Common Acid/Base Indicators
– The list of acid/base indicators is large and includes many organic compounds.
– Indicators are available for almost any desired pH range.
– A few common indicators and their properties are listed in the following table:
– Note that the transition ranges vary from 1.1 to 2.2, with the average being about 1.6 units.
Reference
- Modern analytical chemistry / David Harvey / The McGraw-Hill Companies, Inc./ , 2000 . USA
- Dean’s Analytical Chemistry Handbook / Pradyot Patnaik / The McGraw-Hill Companies, 2nd Editionm, 2004 .USA
- Fundamentals of analytical chemistry / Douglas A. Skoog, Donald M. West, F. James Holler, Stanley R. Crouch. (ninth edition) , 2014 . USA