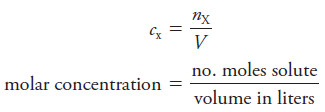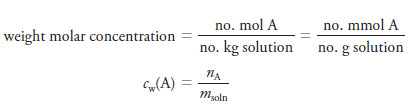Gravimetric Titrations | Definition, Calculations & Advantages
Gravimetric titrations
– Mass (weight) or gravimetric titrations differ from their volumetric counterparts in that the mass of titrant is measured rather than the volume.
– Therefore, in a mass titration, a balance and a weighable solution dispenser are substituted for a buret and its markings.
– Gravimetric titrations actually predate volumetric titrations by more than 50 years.
– With the advent of reliable burets, however, mass titrations were largely supplanted by volumetric methods because the former required relatively elaborate equipment and were tedious and time consuming.
– The availability of sensitive, low-cost, top-loading digital analytical balances and convenient plastic solution dispensers has changed this situation completely, and mass titrations can now be performed as easily and rapidly as volumetric titrations.
Calculations Associated with Mass Titrations
– The most common way to express concentration for mass titrations is the weight concentration, cw, in weight molar concentration units, Mw, which is the number of moles of a reagent in one kilogram of solution or the number of millimoles in one gram of solution.
– Thus, aqueous 0.1 Mw NaCl contains 0.1 mol of the salt in 1 kg of solution or 0.1 mmol in 1 g of the solution.
– The weight molar concentration cw(A) of a solution of a solute A is computed using either one of two equations that are analogous to Equation :
where nA is the number of moles of species A and msoln is the mass of the solution.
Advantages of Gravimetric Titrations
– In addition to greater speed and convenience, mass titrations offer certain other
advantages over their volumetric counterparts:
(1) Calibration of glassware and tedious cleaning to ensure proper drainage are completely eliminated.
(2) Temperature corrections are unnecessary because the mass (weight) molar concentration does not change with temperature, in contrast to the volume molar concentration.
– This advantage is particularly important in nonaqueous titrations because of the high coefficients of expansion of most organic liquids (about 10 times that of water).
(3) Mass measurements can be made with considerably greater precision and accuracy than can volume measurements.
– For example, 50 g or 100 g of an aqueous solution can be readily measured to 61 mg, which corresponds to 60.001 mL.
– This greater sensitivity makes it possible to choose sample sizes that lead to significantly smaller consumption of standard reagents.
(4) Gravimetric titrations are more easily automated than are volumetric titrations.
Reference
- Modern analytical chemistry / David Harvey / The McGraw-Hill Companies, Inc./ , 2000 . USA
- Dean’s Analytical Chemistry Handbook / Pradyot Patnaik / The McGraw-Hill Companies, 2nd Editionm, 2004 .USA
- Fundamentals of analytical chemistry / Douglas A. Skoog, Donald M. West, F. James Holler, Stanley R. Crouch. (ninth edition) , 2014 . USA