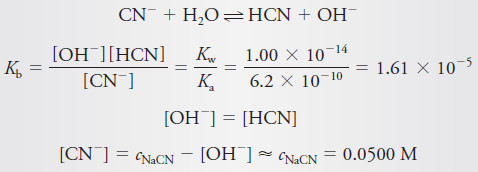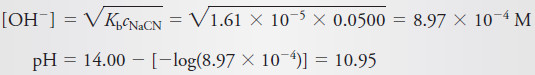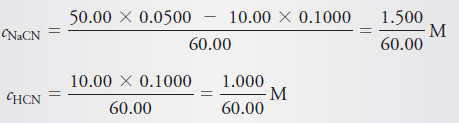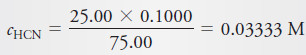Titration Curves for Weak Bases
– In this topic, we will discuss The titration Curves for Weak Bases.
Titration Curves for Weak Bases
– The calculations needed to draw the titration curves for weak bases are analogous to those of a weak acid, as shown in The following Example .
A 50.00-mL aliquot of 0.0500 M NaCN (Ka for HCN = 6.2 × 10-10) is titrated with 0.1000 M HCl. The reaction is
CN– + H3O+ ↔ HCN + H2O
Calculate the pH after the addition of (a) 0.00, (b) 10.00, (c) 25.00, and (d) 26.00 mL of acid.
Solution
(a) 0.00 mL of Reagent
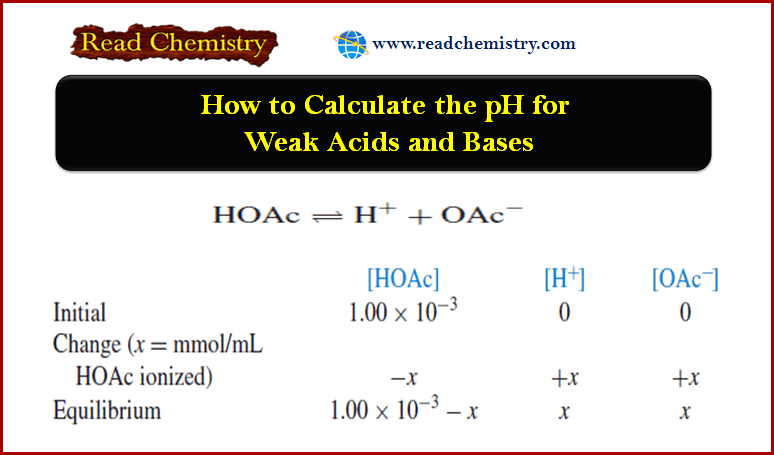
– The pH of a solution of NaCN can be calculated by this method:
– Substituting into the dissociation-constant expression gives, after rearranging,
(b) 10.00 mL of Reagent
– Addition of acid produces a buffer with a composition given by
– These values are then substituted into the expression for the acid dissociation constant of HCN to give [H3O+] directly :
(c) 25.00 mL of Reagent
– This volume corresponds to the equivalence point, where the principal solute species is the weak acid HCN. Thus,
(d) 26.00 mL of Reagent
– The excess of strong acid now present suppresses the dissociation of the HCN to the point where its contribution to the pH is negligible. Thus,
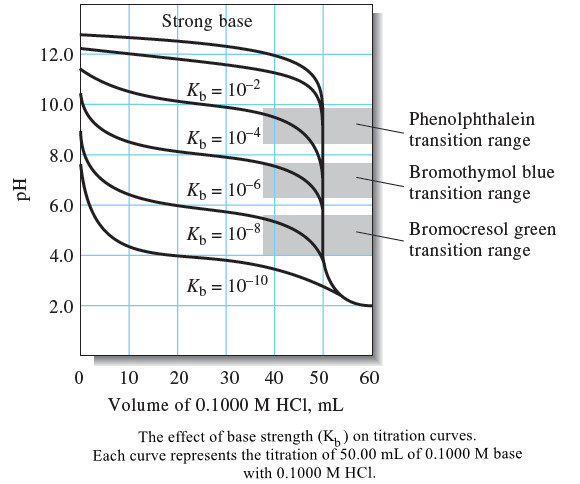
– The following Figure shows hypothetical titration curves for a series of weak bases of different strengths.
– The curves show that indicators with mostly acidic transition ranges must be used for weak bases.
Determining the pK Values for Amino Acids
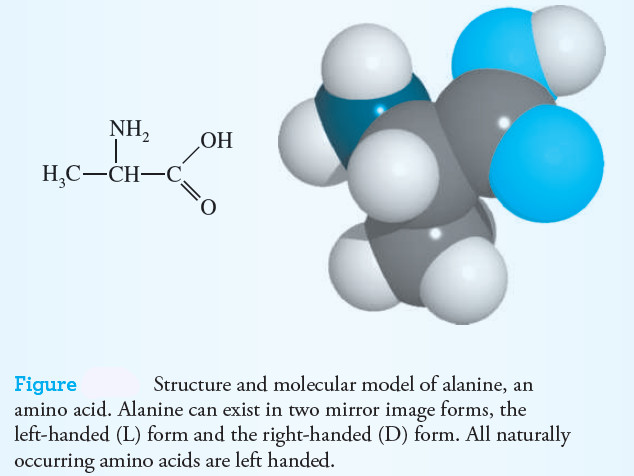
– Amino acids contain both an acidic and a basic group.
– For example, the structure of alanine is represented in the following figure.
– The amine group behaves as a base, and at the same time the carboxyl group acts as an acid.
– In aqueous solution, the amino acid is an internally ionized molecule, or “zwitterion,” in which the amine group acquires a proton and becomes positively charged while the carboxyl group, having lost a proton becomes negatively charged.
– Since the zwitterion has both acidic and basic character, two pKs can be determined.
– The pK for deprotonation of the protonated amine group can be determined by adding base, while the pK for protonating the carboxyl group can be determined by adding acid.
– In practice, a solution is prepared containing a known concentration of the amino acid. Hence, the investigator knows the amount of base or acid to add to reach halfway to the equivalence point.
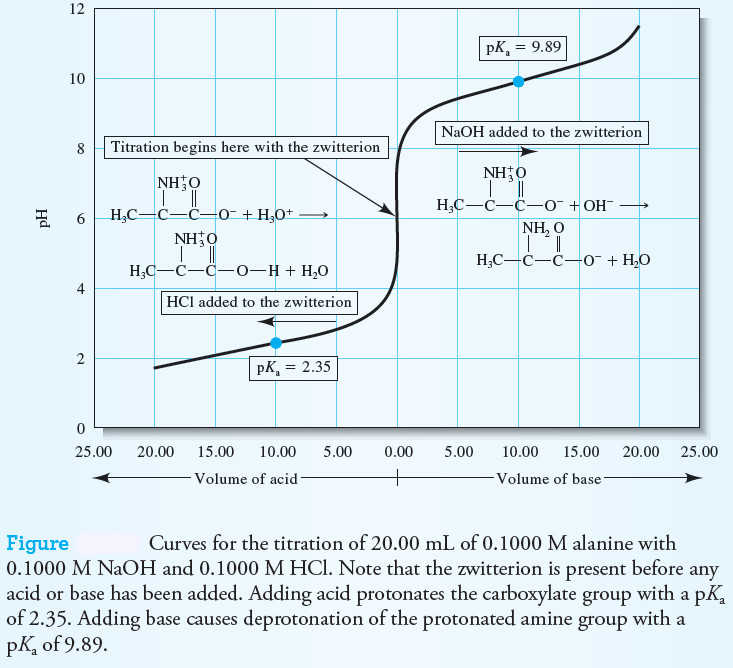
– A curve of pH versus volume of acid or base added is shown in the following Figure.
– In this type of experiment, the titration starts in the middle of the plot (0.00 mL added) and, for determining pK values, is only taken to a point that is half the volume required for equivalence.
– Note in this example for alanine, a volume of 20.00 mL of HCl is needed to completely protonate the carboxyl group.
– By adding acid to the zwitterion, the curve to the left of 0.00 volume is obtained.
– At a volume of 10.00 mL of HCl added, the pH is equal to the pKa for the carboxyl group, 2.35.
– By adding NaOH to the zwitterion, the pK for deprotonating the NH3+ group can be determined.
– Now, 20.00 mL of base is required for complete deprotonation.
– At a volume of 10.00 mL of NaOH added, the pH is equal to the pKa for the amine group, or 9.89.
– The pKa values for other amino acids and more complicated biomolecules such as peptides and proteins can often be obtained in a similar manner.
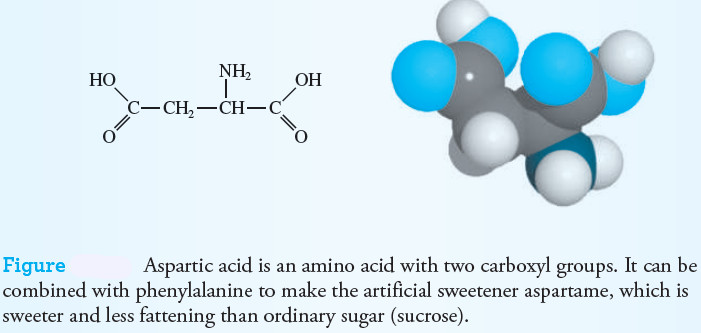
– Some amino acids have more than one carboxyl or amine group. Aspartic acid is an example
– It is important to note that in general amino acids cannot be quantitatively determined by direct titration because end points for completely protonating or deprotonating the zwitterion are often indistinct.
– Amino acids are normally determined by high performance liquid chromatography or spectroscopic methods.
Reference
- Modern analytical chemistry / David Harvey / The McGraw-Hill Companies, Inc./ , 2000 . USA
- Dean’s Analytical Chemistry Handbook / Pradyot Patnaik / The McGraw-Hill Companies, 2nd Editionm, 2004 .USA
- Fundamentals of analytical chemistry / Douglas A. Skoog, Donald M. West, F. James Holler, Stanley R. Crouch. (ninth edition) , 2014 . USA