Some Terms Used in Volumetric Titration
Some Terms Used in Volumetric Titration
– A standard solution (or a standard titrant) is a reagent of known concentration that is used to carry out a volumetric titration.
– The volumetric titration is performed by slowly adding a standard solution from a buret or other liquid dispensing device to a solution of the analyte until the reaction between the two is judged complete.
– The volume or mass of reagent needed to complete the titration is determined from the difference between the initial and final readings.
– A volumetric titration process is depicted in the next title.
– It is sometimes necessary to add an excess of the standard titrant and then determine the excess amount by back-titration with a second standard titrant.
– For example, the amount of phosphate in a sample can be determined by adding a measured excess of standard silver nitrate to a solution of the sample, which leads to the formation of insoluble silver phosphate:
3Ag+ + PO43- → Ag3PO4 (s)
– The excess silver nitrate is then back-titrated with a standard solution of potassium thiocyanate:
Ag+ + SCN– → AgSCN(s)
– The amount of silver nitrate is chemically equivalent to the amount of phosphate ion plus the amount of thiocyanate used for the back-titration.
– The amount of phosphate is then the difference between the amount of silver nitrate and the amount of thiocyanate.
Volumetric Titration process
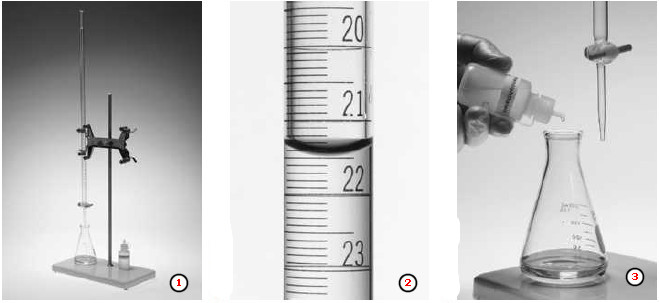
(1) Typical setup for carrying out a titration.
– The apparatus consists of a buret, a buret stand and clamp with a white porcelain base to provide an appropriate background for viewing indicator changes, and a wide-mouth Erlenmeyer flask containing a precisely known volume of the solution to be titrated.
– The solution is normally delivered into the flask using a pipet.
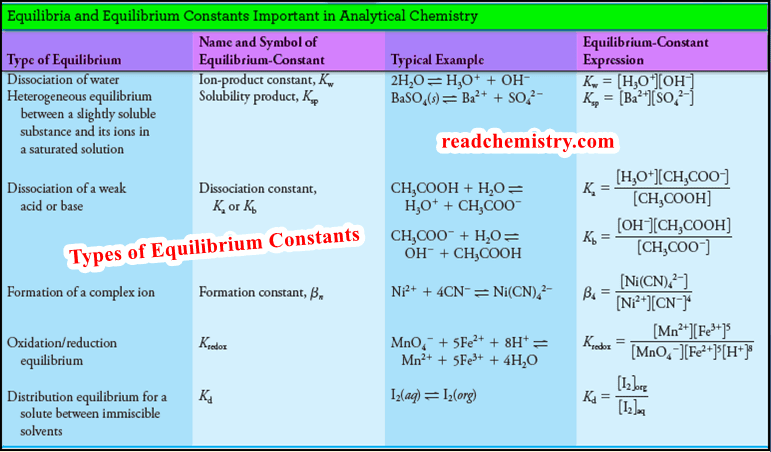
(2) Detail of the buret graduations.
– Normally, the buret is filled with titrant solution to within 1 or 2 mL of the zero position at the top.
– The initial volume of the buret is read to the nearest 0.01 mL.
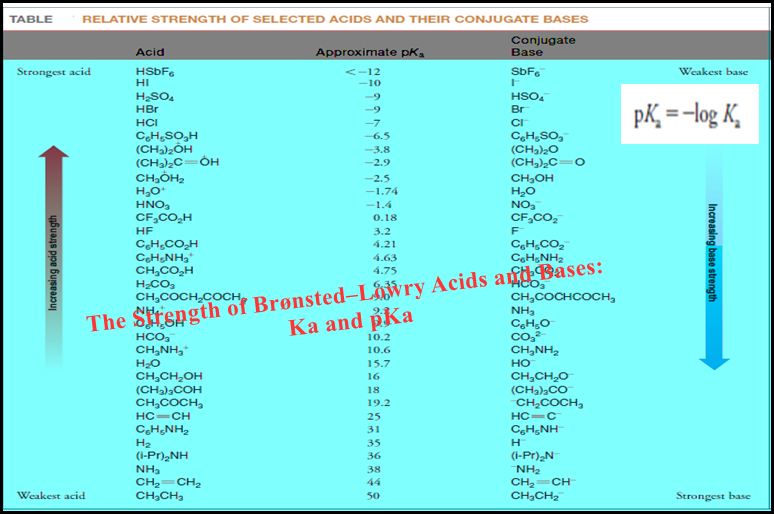
(3) Before the titration begins.
– The solution to be titrated, an acid in this example, is placed in the flask, and the indicator is added as shown in the photo.
– The indicator in this case is phenolphthalein, which turns pink in basic solution.
(4) During titration.
– The titrant is added to the flask with swirling until the color of the indicator persists.
– In the initial region of the titration, titrant may be added rather rapidly, but as the end point is approached, increasingly smaller portions are added; at the end point, less than half a drop of titrant should cause the indicator to change color.
(5) Titration end point.
– The end point is achieved when the barely perceptible pink color of phenolphthalein persists.
– The flask on the left shows the titration less than half a drop prior to the end point; the middle flask shows the end point.
– The final reading of the buret is made at this point, and the volume of base delivered in the titration is calculated from the difference between the initial and final buret readings.
– The flask on the right shows what happens when a slight excess of base is added to the titration mixture.
– The solution turns a deep pink color, and the end point has been exceeded.
– In color plate 9, the color change at the end point is much easier to see than in this black-and- white version
Equivalence Points and End Points
– The equivalence point in a titration is a theoretical point reached when the amount of added titrant is chemically equivalent to the amount of analyte in the sample.
– For example, the equivalence point in the titration of sodium chloride with silver nitrate occurs after exactly one mole of silver ion has been added for each mole of chloride ion in the sample.
– The equivalence point in the titration of sulfuric acid with sodium hydroxide is reached after introducing 2 moles of base for each mole of acid.
– We cannot determine the equivalence point of a titration experimentally. Instead, we can only estimate its position by observing some physical change associated with the condition of chemical equivalence.
– The position of this change is called the end point for the titration.
– We try very hard to ensure that any volume or mass difference between the equivalence point and the end point is small.
– Such differences do exist, however, as a result of inadequacies in the physical changes and in our ability to observe them.
– The difference in volume or mass between the equivalence point and the end point is the titration error.
– Indicators are often added to the analyte solution to produce an observable physical change (signaling the end point) at or near the equivalence point.
– Large changes in the relative concentration of analyte or titrant occur in the equivalence-point region.
– These concentration changes cause the indicator to change in appearance.
– Typical indicator changes include the appearance or disappearance of a color, a change in color, or the appearance or disappearance of turbidity.
– As an example, the indicator used in the neutralization titration of hydrochloric acid with sodium hydroxide is phenolphthalein, which causes the solution to change from colorless to a pink color once excess sodium hydroxide has been added.
– We often use instruments to detect end points. These instruments respond to properties of the solution that change in a characteristic way during the titration.
– Among such instruments are colorimeters, turbidimeters, spectrophotometers, temperature monitors, refractometers, voltmeters, current meters, and conductivity meters.
Primary Standards
– A primary standard is a highly purified compound that serves as a reference material in titrations and in other analytical methods.
– The accuracy of a method critically depends on the properties of the primary standard.
– Important requirements for a primary standard are the following:
(1) High purity. Established methods for confirming purity should be available.
(2) Atmospheric stability.
(3) Absence of hydrate water so that the composition of the solid does not change with variations in humidity.
(4) Modest cost.
(5) Reasonable solubility in the titration medium.
(6) Reasonably large molar mass so that the relative error associated with weighing the standard is minimized.
– Very few compounds meet or even approach these criteria, and only a limited number of primary standard substances are available commercially.
– As a consequence, less pure compounds must sometimes be used in place of a primary standard.
– The purity of such a secondary standard must be established by careful analysis.
Standard Solutions
– Standard solutions play a central role in all titrations.
– Therefore, we must consider the desirable properties for such solutions, how they are prepared, and how their concentrations are expressed.
– The ideal standard solution for a titrimetric method will:
(1) be sufficiently stable so that it is necessary to determine its concentration only once;
(2) react rapidly with the analyte so that the time required between additions of reagent is minimized;
(3) react more or less completely with the analyte so that satisfactory end points are realized;
(4) undergo a selective reaction with the analyte that can be described by a balanced equation.
– Few reagents completely meet these ideals.
– The accuracy of a titration can be no better than the accuracy of the concentration
of the standard solution used.
– Two basic methods are used to establish the concentration of such solutions.
– The first is the direct method in which a carefully determined mass of a primary standard is dissolved in a suitable solvent and diluted to a known volume in a volumetric flask.
– The second is by standardization in which the titrant to be standardized is used to titrate:
(1) a known mass of a primary standard,
(2) a known mass of a secondary standard, or
(3) a measured volume of another standard solution.
– A titrant that is standardized is sometimes referred to as a secondarystandard solution.
– The concentration of a secondary-standard solution is subject to a larger uncertainty than is the concentration of a primary-standard solution.
– If there is a choice, then, solutions are best prepared by the direct method.
– Many reagents, however, lack the properties required for a primary standard and, therefore, require standardization.
Conclusion of Terms Used in Volumetric Titration
– Titration methods are based on determining the quantity of a reagent of known concentration that is required to react completely with the analyte. The reagent may be a standard solution of a chemical or an electric current of known magnitude.
– In volumetric titration, the volume of a standard reagent is the measured quantity.
– In coulometric titrations, the quantity of charge required to complete a reaction with the analyte is the measured quantity.
– A standard solution is a reagent of known concentration. Standard solutions are used in titrations and in many other chemical analyses.
– Back-titration is a process in which the excess of a standard solution used to consume an analyte is determined by titration with a second standard solution.
– Back-titrations are often required when the rate of reaction between the analyte and reagent is slow or when the standard solution lacks stability.
– The equivalence point is the point in a titration when the amount of added standard reagent is equivalent to the amount of analyte.
– The end point is the point in a titration when a physical change occurs that is associated with the condition of chemical equivalence.
– In volumetric methods, the titration error, Et, is given by:
Et = Vep + Veq
where Vep is the actual volume of reagent required to reach the end point and Veq is the theoretical volume necessary to reach the equivalence point
– A primary standard is an ultrapure compound that serves as the reference material for a titration or for another type of quantitative analysis.
– A secondary standard is a compound whose purity has been determined by chemical analysis. The secondary standard serves as the working standard material for titrations and for many other analyses.
Reference
- Modern analytical chemistry / David Harvey / The McGraw-Hill Companies, Inc./ , 2000 . USA
- Dean’s Analytical Chemistry Handbook / Pradyot Patnaik / The McGraw-Hill Companies, 2nd Editionm, 2004 .USA
- Fundamentals of analytical chemistry / Douglas A. Skoog, Donald M. West, F. James Holler, Stanley R. Crouch. (ninth edition) , 2014 . USA