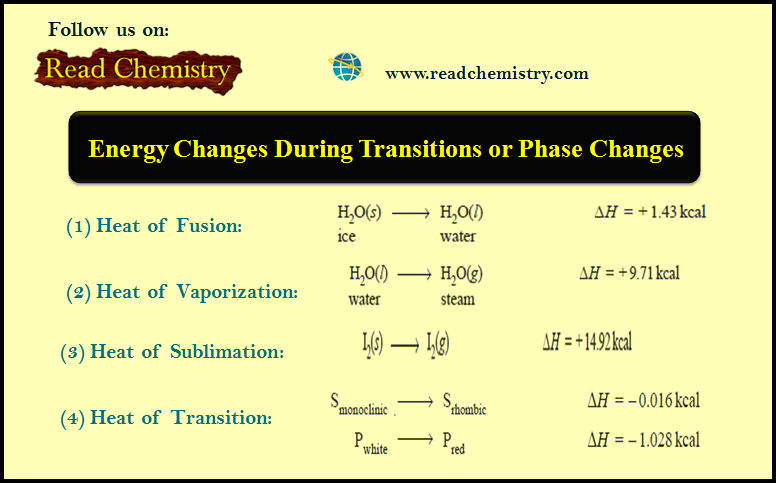
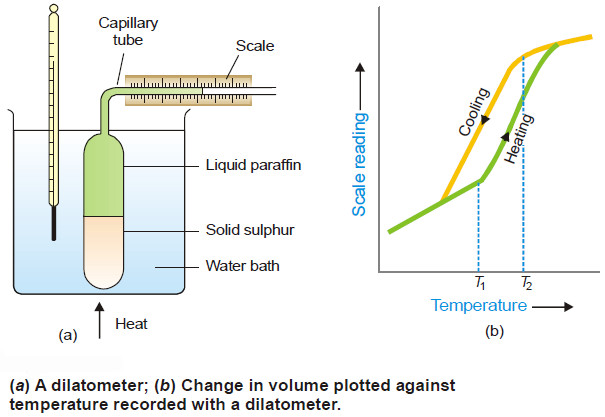
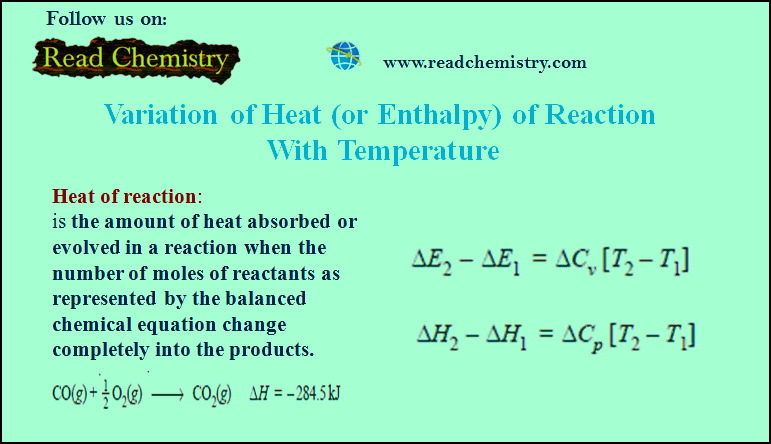
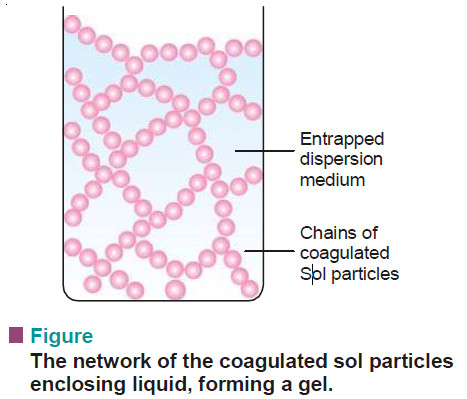
Associated Colloids
– In this subject, we will discuss the Associated Colloids.
Associated Colloids
– The molecules of substances as soaps and artificial detergents are smaller than the colloidal particles. However in concentrated solutions these molecules form aggregates of colloidal size.
– Substances whose molecules aggregate spontaneously in a given solvent to form particles of colloidal dimensions are called Associated colloids or Association Colloids sols.
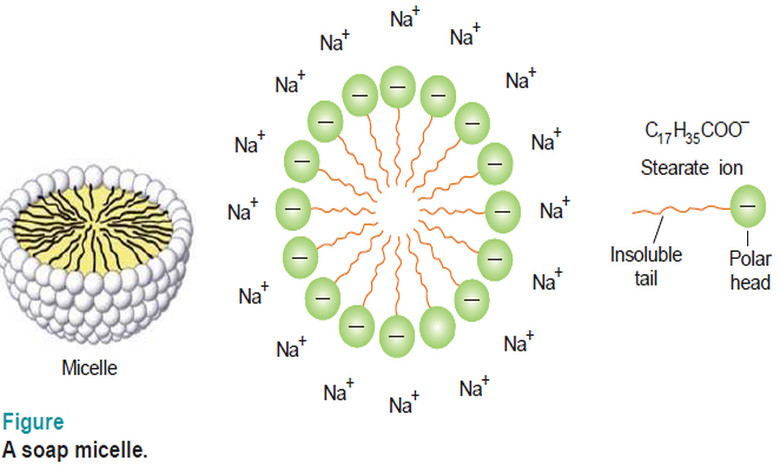
– The colloidal aggregates of soap or detergent molecules formed in the solvent are referred to as micelles.
– Some examples of associated colloids are :
Explanation
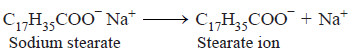
– Soap or detergent molecule ionises in water to form an anion and sodium ion.
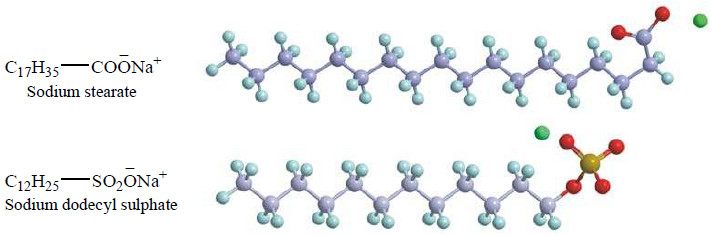
– Thus sodium stearate (a typical soap) furnishes stearate anion and sodium ion in aqueous solution.
– As many as 70 stearate ions aggregate to form a micelle of colloidal size.
– The stearate ion has a long hydrocarbon chain (17 carbons) with a polar —COO– group at one end.
– The zig zag hydrocarbon tail is shown by a wavy line and the polar head by a hollow circle.
– In the micelle formation, the tails being insoluble in water are directed toward the centre, while the soluble polar heads are on the surface in contact with water (seeFig above).
– The charge on the micelle due to the polar heads accounts for the stability of the particle.
Cleansing Action of Soaps and Detergents
The cleansing action of soap is due to:
(1) Solubilisation of grease into the micelle
(2) Emulsification of grease
Solubilisation
– In relatively strong solution the soap (or detergent) anions spontaneously form a micelle.
– The hydrocarbon tails are in the interior of the micelle and COO– ions on the surface.
– The grease stain is thus absorbed into the interior of the micelle which behaves like liquid hydrocarbons.
– As the stain is detached from the fabric, the dirt particles sticking to the stain are also removed.
Emulsification
– As already discussed the soap or detergent molecules are ionised in water.
– The anions are made of oil-soluble hydrocarbon tails and water-soluble polar heads. Thus soap anion has a long hydrocarbon tail with a polar head, —COO–.
– When soap solution is added to a fabric, the tails of the soap anions are pegged into the grease stain.
– The polar heads protrude from the grease surface and form a charged layer around it. Thus by mutual repulsions the grease droplets are suspended in water.
– The emulsified grease stains are washed away with soap solution.