Time Dependence of NMR Spectroscopy
– In this topic, we will discuss The Time Dependence of NMR Spectroscopy.
Time Dependence of NMR Spectroscopy
– We have already seen evidence that NMR does not provide an instantaneous picture of a molecule.
– For example, a terminal alkyne does not give a spectrum where the molecules oriented along the field absorb at a high field and those oriented perpendicular to the field absorb at a lower field.
– What we see is one signal whose position is averaged over the chemical shifts of all the orientations of a rapidly tumbling molecule.
– In general, any type of movement or change that takes place faster than about a hundredth of a second will produce an averaged NMR spectrum.
Conformational Changes
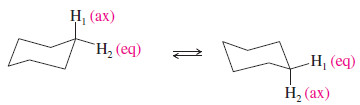
– This principle is illustrated by the cyclohexane spectrum.
– In the chair conformation, there are two kinds of protons: the axial hydrogens and the equatorial hydrogens.
– The axial hydrogens become equatorial and the equatorial hydrogens become axial by chair–chair interconversions.
– These interconversions are fast on an NMR time scale at room temperature.
– The NMR spectrum of cyclohexane shows only one sharp, averaged peak (at δ 1.4) at room temperature.
– Low temperatures retard the chair–chair interconversion of cyclohexane.
– The NMR spectrum at -89 oC shows two nonequivalent types of protons that split each other, giving two broad bands corresponding to the absorptions of the axial and equatorial protons.
– The broadening of the bands results from spin-spin splitting between axial and equatorial protons on the same carbon atom and on adjacent carbons.
– This technique of using low temperatures to stop conformational interconversions is called freezing out the conformations.
Fast Proton Transfers
Hydroxyl Protons
– Like conformational interconversions, chemical processes often occur faster than the NMR technique can observe them.
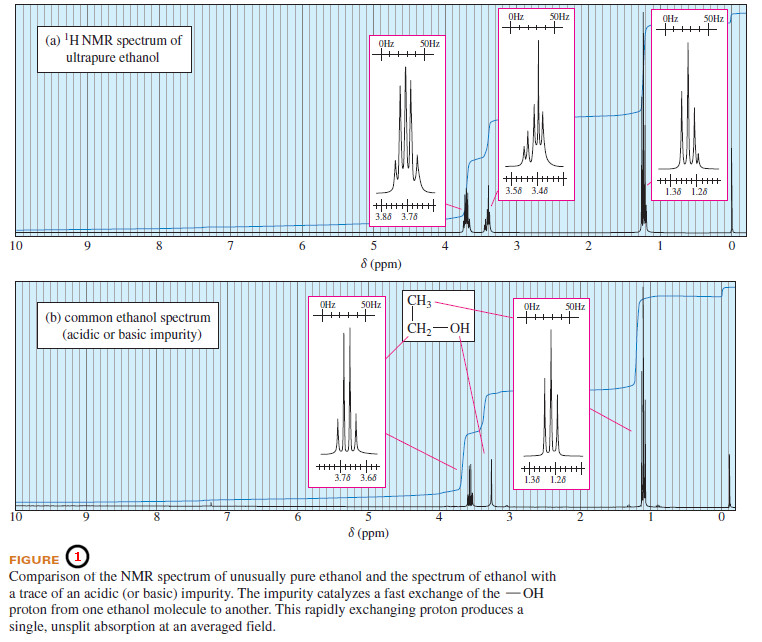
– Figure (1) shows two NMR spectra for ethanol.
– Figure 1(a) shows coupling between the hydroxyl (-OH) proton and the adjacent methylene (-CH2-) protons, with a coupling constant of about 5 Hz.
– This is an ultrapure sample of ethanol with no contamination of acid, base, or water.
– Part (b) shows a typical sample of ethanol, with some acid or base present to catalyze the interchange of the hydroxyl protons.
– No splitting is seen between the hydroxyl proton and the methylene protons.
– During the NMR measurement, each hydroxyl proton becomes attached to a large number of different ethanol molecules and experiences all possible spin arrangements of the methylene group.
– What we see is a single, unsplit hydroxyl absorption corresponding to the averaged field the proton experiences from bonding to many different ethanol molecules.
– Proton exchange occurs in most alcohols and carboxylic acids, and in many amines and amides.
– If the exchange is fast (as it usually is for -OH protons), we see one sharp averaged signal.
– If the exchange is very slow, we see splitting.
– But If the exchange is moderately slow, we may see a broadened peak that is neither cleanly split nor cleanly averaged.
N-H Protons
– Protons on nitrogen often show broadened signals in the NMR, both because of moderate rates of exchange and because of the magnetic properties of the nitrogen nucleus.
– Depending on the rate of exchange and other factors, N-H protons may give absorptions that are sharp and cleanly split, sharp and unsplit (averaged), or broad and shapeless.
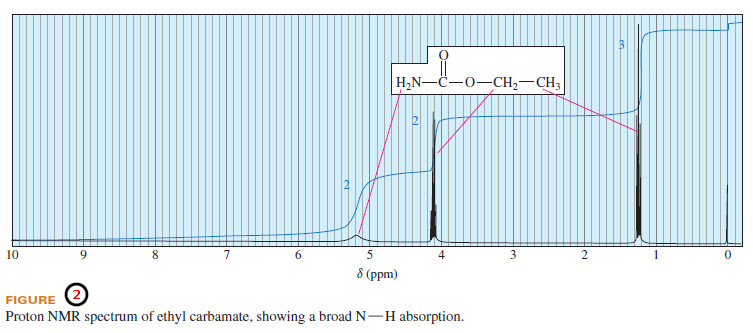
– Figure (2) illustrates an NMR spectrum where the -NH2 protons produce a very broad absorption, the shapeless peak centered at δ 5.2.
– Because the chemical shifts of O-H and N-H protons depend on the concentration and the solvent, it is often difficult to tell whether or not a given peak corresponds to one of these types of protons.
– We can use proton exchange to identify their NMR signals by shaking the sample with an excess of deuterium oxide,D2O.
– Any exchangeable hydrogens are quickly replaced by deuterium atoms, which are invisible in the proton NMR spectrum.
R-O-H + D-O-D ↔ R-O-D + D-O-H
R-NH2 + 2 D-O-D ↔ R-ND2 + 2 D-O-H
– When a second NMR spectrum is recorded (after shaking with D2O), the signals from any exchangeable protons are either absent or much less intense.
References:
- Organic chemistry / L.G. Wade, Jr / 8th ed, 2013 / Pearson Education, Inc. USA.
- Fundamental of Organic Chemistry / John McMurry, Cornell University/ 8th ed, 2016 / Cengage Learningm, Inc. USA.
- Organic Chemistry / T.W. Graham Solomons, Craig B. Fryhle , Scott A. Snyder / 11 ed, 2014/ John Wiley & Sons, Inc. USA.