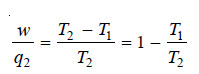Laws of thermodynamics
Zeroth law of thermodynamics
– The zeroth law of thermodynamics is a generalized statement about bodies in contact at thermal equilibrium and is the basis for the concept of temperature.
– The most common definition of the zeroth law of thermodynamics is: If two thermodynamic systems are in thermal equilibrium with a third, they are also in thermal equilibrium with each other.
– The term zeroth law was coined by Ralph H. Fowler.
– In many ways, the law is more fundamental than any of the others. However, the need to state it explicitly as a law was not perceived until the first third of the 20th century, long after the first three laws were already widely in use and named as such, hence the zero numbering. There is still some discussion about its status in relation to the other three laws.
– A system in thermal equilibrium is a system whose macroscopic properties (like pressure, temperature, volume, etc.) are not changing in time.
– A hot cup of coffee sitting on a kitchen table is not at equilibrium with its surroundings because it is cooling off and decreasing in temperature.
– Once its temperature stops decreasing, it will be at room temperature, and it will be in thermal equilibrium with its surroundings.
– Two systems are said to be in thermal equilibrium when:
(a) both of the systems are in a state of equilibrium, and
(b) they remain so when they are brought into contact, where ‘contact’ is meant to imply the possibility of exchanging heat, but not work or particles.
– And more generally, two systems can be in thermal equilibrium without thermal contact if one can be certain that if they were thermally connected, their properties would not change in time. Thus, thermal equilibrium is a relation between thermodynamical systems.
Mathematically, the zeroth law expresses that this relation is an equivalence relation.
First law of thermodynamics
– The first law of thermodynamics is, in fact, an application of the broad principle known as the Law of Conservation of Energy to the thermodynamic system.
– The first law of thermodynamics states that : the total energy of an isolated system remains constant though it may change from one form to another.
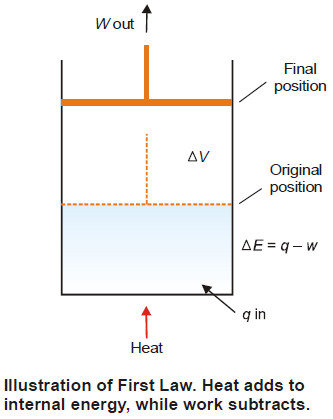
– When a system is changed from state A to state B, it undergoes a change in the internal energy from EA to EB. Thus, we can write
ΔE = EB – EA
– This energy change is brought about by the evolution or absorption of heat and/or by work being done by the system.
– Because the total energy of the system must remain constant, we can write the mathematical statement of the First Law as :
ΔE = q – w …(1)
– where q = the amount of heat supplied to the system, w = work done by the system
– The First Law may also be stated as : the net energy change of a closed system is equal to the heat transferred to the system minus the work done by the system
– To illustrate the mathematical statement of the First Law, let us consider the system ‘expanding hot gas’.
– The gas expands against an applied constant pressure by volume ΔV.
– The total mechanical work done is given by the relation
w = P × ΔV …(2)
From (1) and (2), we can restate
ΔE = q – P × ΔV
Other Definitions of First Law
(1) Whenever energy of a particular type disappears equivalent amount of another type must be produced.
(2) Total energy of a system and surroundings remains constant (or conserved)
(3) It is impossible to construct a perpetual motion machine that can produce work without spending energy on it.
Second law of thermodynamics
– The second law of thermodynamics states that : whenever a spontaneous process takes place, it is accompanied by an increase in the total energy of the universe.
– More specifically, we take the term ‘universe’ to mean the system and the surroundings. Thus,
ΔSuniv = ΔSsyst + ΔSsurr
– The second law, as stated above, tells us that when an irreversible spontaneous process occurs, the entropy of the system and the surroundings increases. In other words ΔSuniv > 0.
– When a reversible process occurs, the entropy of the system remains constant. ΔSuniv = 0. Since the entire universe is undergoing spontaneous change, the second law can be most generally and concisely stated as : the entropy of the system is constantly increasing.
More statements of the Second law
– From equation:
– Evidently w/q2 is less than 1, or q2 is greater than w. This means that heat transferred by a
spontaneous process is never completely converted into work (If so, w/q2 would be 1).
– This leads to another statement of the Second law (Lord Kelvin): It is impossible to take heat from a hotter reservoir and convert it completely into work by a cyclic process without transferring a part of heat to a cooler reservoir.
This statement recognises the fact that heat engines could never be 100% efficient, since some heat must be returned to a low-temperature reservoir.
– Another statement of the Second law was given by Clausius: It is impossible for a cyclic process to transfer heat from a body at a lower temperature to one at higher temperature without at the same time converting some work to heat.
– This statement recognises that heat flows spontaneously from hot objects to cold objects and to get it flow in the opposite direction, we have to expend some work.
Third law of thermodynamics
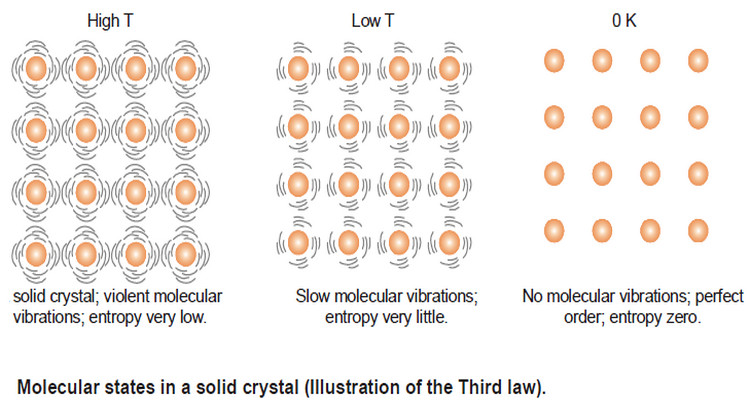
– The entropy of a substance varies directly with temperature.
– The lower the temperature, the lower the entropy.
– For example, water above 100ºC at one atmosphere exists as a gas and has higher entropy (higher disorder).
– The water molecules are free to roam about in the entire container.
– When the system is cooled, the water vapour condenses to form a liquid.
– Now the water molecules are confined below the liquid level but still can move about somewhat freely.
– Thus the entropy of the system has decreased. On further cooling, water molecules join together to form ice crystal.
– The water molecules in the crystal are highly ordered and entropy of the system is very low.
– If we cool the solid crystal still further, the vibration of molecules held in the crystal lattice gets slower and they have very little freedom of movement (very little disorder) and hence very small entropy.
– Finally, at absolute zero all molecular vibration ceases and water molecules are in perfect order. Now the entropy of the system will be zero.
– This leads us to the statement of the third law of thermodynamics : at absolute zero, the entropy of a pure crystal is also zero. That is, S = 0 at T = 0 K.
References
- Atkins’ Physical Chemistry / Peter Atkin, Julio de Paula, James Keeler / 12th edition, 2022 / Oxford University Press, UK.
- Physical Chemistry/ Robert G. Mortimer/ 3rd Edition / 2008/ Elsevier Inc, USA.
- Essentials of Physical Chemistry /Arun Bahl, B.S Bahl and G.D. Tuli / multicolor edition/ S. Chand Publishing co / india.
- Physical chemistry for the chemical sciences / Raymond Chang, John W. Thoman, Jr./1st edition, 2014/ University Science Books, USA