Ions and ionic compounds
– In this topic, we will discuss definition of The Ions and ionic compounds
Ions and ionic compounds
– So far we have discussed only compounds that exist as discrete molecules.
– Some compounds, such as sodium chloride, NaCl, consist of collections of large numbers of ions.
– An ion is an atom or group of atoms that carries an electric charge.
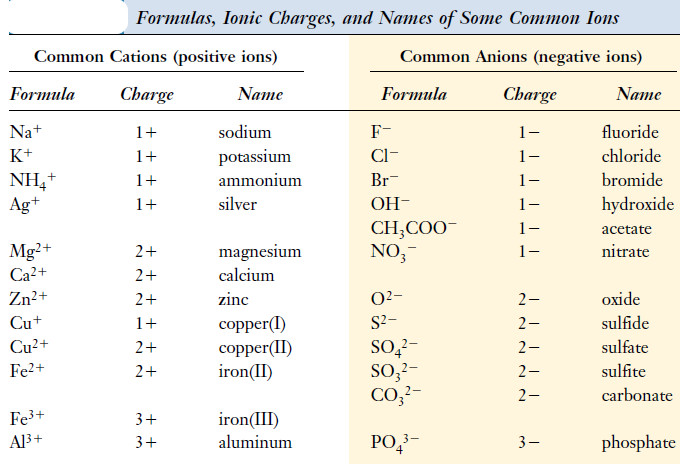
– Ions that possess a positive charge, such as the sodium ion, Na+, are called cations.
– Those carrying a negative charge, such as the chloride ion, Cl–, are called anions.
– The charge on an ion must be included as a superscript on the right side of the chemical symbol(s) when we write the formula for the individual ion.
– an atom consists of a very small, very dense, positively charged nucleus surrounded by a diffuse distribution of negatively charged particles called electrons.
– The number of positive charges in the nucleus defines the identity of the element to which the atom corresponds.
– Electrically neutral atoms contain the same number of electrons outside the nucleus as positive charges (protons) within the nucleus.
– Ions are formed when neutral atoms lose or gain electrons.
– An Na+ ion is formed when a sodium atom loses one electron, and a Cl– ion is formed when a chlorine atom gains one electron.
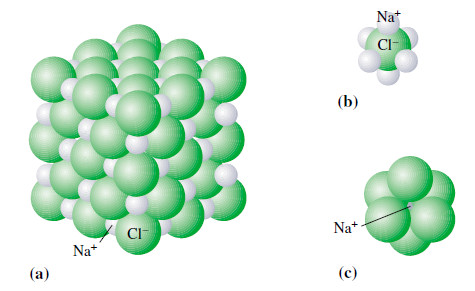
– The compound NaCl consists of an extended array of Na+ and Cl– ions (Figure).
– Within the crystal (though not on the surface) each Na+ ion is surrounded at equal distances by six Cl– ions, and each Cl– ion is similarly surrounded by six Na+ ions.
– Any compound, whether ionic or molecular, is electrically neutral; that is, it has no net charge.
– In NaCl this means that the Na+ and Cl– ions are present in a 1:1 ratio, and this is indicated by the formula NaCl.
– Because there are no “molecules” of ionic substances, we should not refer to “a molecule of sodium chloride, NaCl,” for example.
– Instead, we refer to a formula unit of NaCl, which consists of one Na+ ion and one Cl– ion.
– Likewise, one formula unit of CaCl2 consists of one Ca2+ ion and two Cl– ions.
– As you will see in the next section, we speak of the formula unit of all ionic compounds as the smallest, whole-number ratios of ions that yield neutral representations. It is also acceptable to refer to a formula unit of a molecular compound.
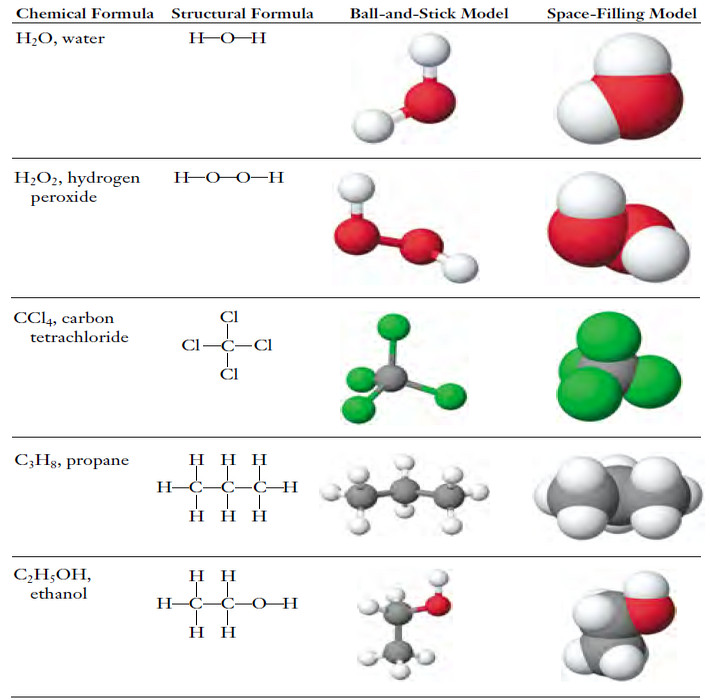
– One formula unit of propane, C3H8, is the same as one molecule of C3H8; it contains three C atoms and eight H atoms bonded together into a group.
– For the present, we shall tell you which substances are ionic and which are molecular when it is important to know.
Polyatomic ions
– Polyatomic ions are groups of atoms that bear an electric charge.
– Examples include the ammonium ion, NH4+ ; the sulfate ion, SO4-2 ; and the nitrate ion, NO3–.
– The following Table shows the formulas, ionic charges, and names of some common ions.
– When writing the formula of a polyatomic compound, we show groups in parentheses when they appear more than once.
– For example, (NH4)2SO4 represents a compound that has two NH4+ ions for each SO42- ion.
– As we shall see, some metals can form more than one kind of ion with a positive charge.
– For such metals, we specify which ion we mean with a Roman numeral—e.g., iron(II) or iron(III).
– Because zinc forms no stable ions other than Zn2+, we do not need to use Roman numerals in its name.
References
- Principles of Inorganic Chemistry / Brian W. Pfennig / 1st ed, 2015 /John Wiley & Sons, Inc/ USA.
- Inorganic Chemistry /Peter Atkins, Tina Overton, Jonathan Rourkel, Mark Weller, Fraser Armstrong, Mike Hagerman / 6th ed, 2014 /W. H. Freeman and Company/ New York, USA.
-