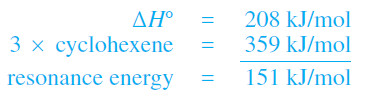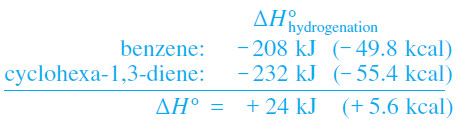The Structure and Properties of Benzene
In this subject, we we will talk about The Discovery of Benzene and The Structure and Properties of Benzene.
The Discovery of Benzene
– In 1825, Michael Faraday isolated a pure compound of boiling point 80 °C from the oily mixture that condensed from illuminating gas, the fuel burned in gaslights.
– Elemental analysis showed an unusually small hydrogen-to-carbon ratio of 1:1, corresponding to an empirical formula of CH. Faraday named the new compound “bicarburet of hydrogen.”
– Eilhard Mitscherlich synthesized the same compound in 1834 by heating benzoic acid, isolated from gum benzoin, in the presence of lime. Like Faraday, Mitscherlich found that the empirical formula was CH.
– He also used a vapor-density measurement to determine a molecular weight of about 78, for a molecular formula of C6H6.
– Since the new compound was derived from gum benzoin, he named it benzin, now called benzene.
– Many other compounds discovered in the nineteenth century seemed to be related to benzene.
– These compounds also had low hydrogen-to-carbon ratios as well as pleasant aromas, and they could be converted to benzene or related compounds.
– This group of compounds was called aromatic because of their pleasant odors. Other organic compounds without these properties were called aliphatic, meaning “fatlike.”
– As the unusual stability of aromatic compounds was investigated, the term aromatic came to be applied to compounds with this stability, regardless of their odors.
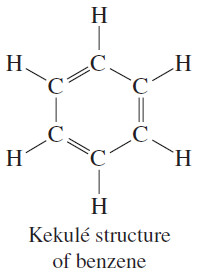
The Kekulé Structure
– In 1866, Friedrich Kekulé proposed a cyclic structure for benzene with three double bonds.
– Considering that multiple bonds had been proposed only recently (1859), the cyclic structure with alternating single and double bonds was considered somewhat bizarre.
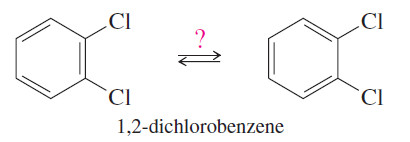
– The Kekulé structure has its shortcomings, however. For example, it predicts two different 1,2 dichlorobenzenes, but only one is known to exist.
– Kekulé suggested (incorrectly) that a fast equilibrium interconverts the two isomers of 1,2-dichlorobenzene.
The Resonance Representation
– The resonance picture of benzene is a natural extension of Kekulé’s hypothesis.
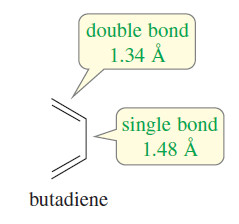
– In a Kekulé structure, the C-C single bonds would be longer than the double bonds.
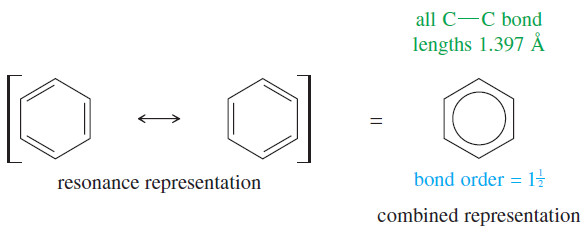
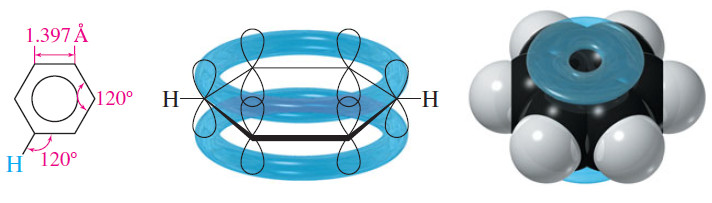
– Spectroscopic methods have shown that the benzene ring is planar and all the bonds are the same length (1.397 Å).
– Because the ring is planar and the carbon nuclei are positioned at equal distances, the two Kekulé structures must differ only in the positioning of the pi electrons.
– Benzene is actually a resonance hybrid of the two Kekulé structures. This representation implies that the pi electrons are delocalized, with a bond order of 3/2 between adjacent carbon atoms.
– The carbon–carbon bond lengths in benzene are shorter than typical single-bond lengths, yet longer than typical double-bond lengths.
– The resonance-delocalized picture explains most of the structural properties of benzene and its derivatives—the benzenoid aromatic compounds.
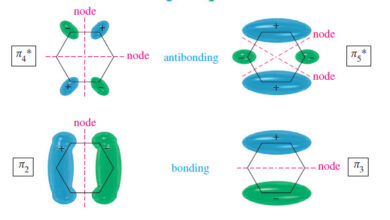
– Because the pi bonds are delocalized over the ring, we often inscribe a circle in the hexagon rather than draw three localized double bonds.
– This representation helps us remember there are no localized single or double bonds, and it prevents us from trying to draw supposedly different isomers that differ only in the placement of double bonds in the ring.
– We often use Kekulé structures in drawing reaction mechanisms, however, to show the movement of individual pairs of electrons.
– Using this resonance picture, we can draw a more realistic representation of benzene.
– Benzene is a ring of six sp2 hybride carbon atoms, each bonded to one hydrogen atom.
– All the carbon–carbon bonds are the same length, and all the bond angles are exactly 120°.
– Each sp2 carbon atom has an unhybridized p orbital perpendicular to the plane of the ring, and six electrons occupy this circle of p orbitals.
At this point, we can define an aromatic compound to be a cyclic compound containing some number of conjugated double bonds and having an unusually large resonance energy.
– Using benzene as the example, we will consider how aromatic compounds differ from aliphatic compounds.
– Then we will discuss why an aromatic structure confers extra stability and how we can predict aromaticity in some interesting and unusual compounds
The Unusual Reactions of Benzene
– Benzene is actually much more stable than we would expect from the simple resonance delocalized picture.
– Both the Kekulé structure and the resonance-delocalized picture show that benzene is a cyclic conjugated triene.
– We might expect benzene to undergo the typical reactions of polyenes.
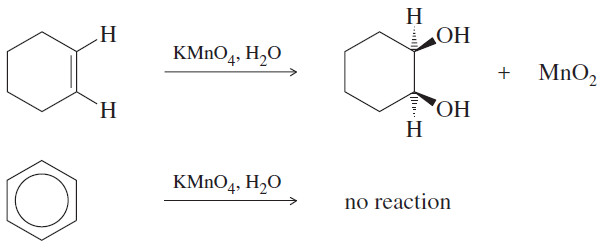
– In fact, its reactions are quite unusual. For example, an alkene decolorizes potassium permanganate by reacting to form a glycol.
– The purple permanganate color disappears, and a precipitate of manganese dioxide forms.
– When permanganate is added to benzene, however, no reaction occurs.
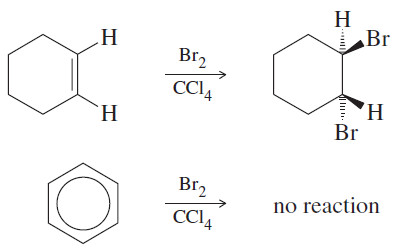
– Most alkenes decolorize solutions of bromine in carbon tetrachloride.
– The red bromine color disappears as bromine adds across the double bond.
– When bromine is added to benzene, no reaction occurs, and the red bromine color remains.
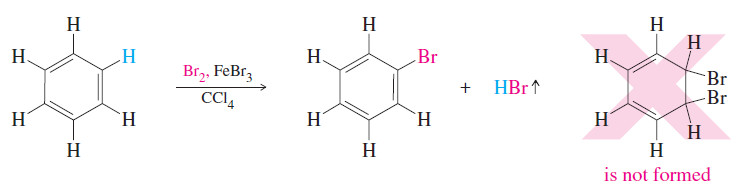
– Addition of a catalyst such as ferric bromide to the mixture of bromine and benzene causes the bromine color to disappear slowly.
– HBr gas is evolved as a by-product, but the expected addition of Br2 does not take place.
– Instead, the organic product results from substitution of a bromine atom for a hydrogen, and all three double bonds are retained.
The Unusual Stability of Benzene
– Benzene’s reluctance to undergo typical alkene reactions suggests that it must be unusually stable.
– By comparing molar heats of hydrogenation, we can get a quantitative idea of its stability.
– Benzene, cyclohexene, and the cyclohexadienes all hydrogenate to form cyclohexane.
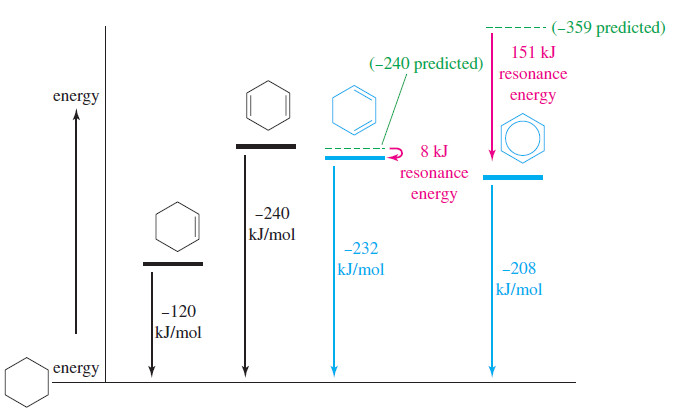
– The following Figure shows how the experimentally determined heats of hydrogenation are used to compute the resonance energies of cyclohexa-1,3-diene and benzene, based on the following reasoning:
(1) Hydrogenation of cyclohexene is exothermic by 120 kJ mol (28.6 kcal mol).
(2) Hydrogenation of cyclohexa-1,4-diene is exothermic by 240 kJ mol (57.4 kcal mol), about twice the heat of hydrogenation of cyclohexene. The resonance energy of the isolated double bonds in cyclohexa-1,4-diene is about zero.
(3) Hydrogenation of cyclohexa-1,3-diene is exothermic by 232 kJ mol (55.4 kcal mol), about 8 kJ (1.8 kcal) less than twice the value for cyclohexene. A resonance energy of 8 kJ (1.8 kcal) is typical for a conjugated diene.
(4) Hydrogenation of benzene requires higher pressures of hydrogen and a more active catalyst. This hydrogenation is exothermic by 208 kJ mol (49.8 kcal mol), about 151 kJ (36.0 kcal) less than 3 times the value for cyclohexene.
– The huge 151 kJ mol (36 kcal mol) resonance energy of benzene cannot be explained by conjugation effects alone.
– The heat of hydrogenation for benzene is actually smaller than that for cyclohexa-1,3-diene.
– The hydrogenation of the first double bond of benzene is endothermic, the first endothermic hydrogenation we have encountered.
– In practice, this reaction is difficult to stop after the addition of 1 mole of H2 because the product (cyclohexa-1,3-diene) hydrogenates more easily than benzene itself. Clearly, the benzene ring is exceptionally unreactive.
Failures of the Resonance Picture
– For many years, chemists assumed that benzene’s large resonance energy resulted from having two identical, stable resonance structures.
– They thought that other hydrocarbons with analogous conjugated systems of alternating single and double bonds would show similar stability.
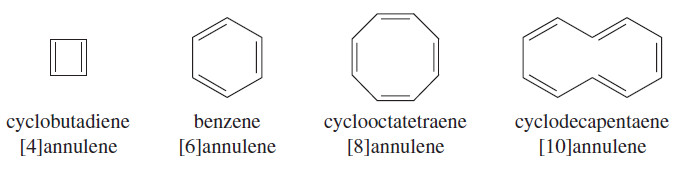
– These cyclic hydrocarbons with alternating single and double bonds are called annulenes.
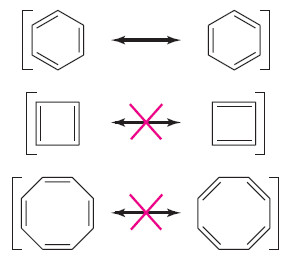
– For example, benzene is the six-membered annulene, so it can be named [6]annulene.
– Cyclobutadiene is [4]annulene, cyclooctatetraene is [8]annulene, and larger annulenes are named similarly.
– For the double bonds to be completely conjugated, the annulene must be planar so the p orbitals of the pi bonds can overlap.
– As long as an annulene is assumed to be planar, we can draw two Kekulé-like structures that seem to show a benzene-like resonance.
– The following Figure 16-3 shows proposed benzene-like resonance forms for cyclobutadiene and cyclooctatetraene.
– Although these resonance structures suggest that the [4] and [8]annulenes should be unusually stable (like benzene), experiments have shown that cyclobutadiene and cyclooctatetraene are not unusually stable. These results imply that the simple resonance picture is incorrect.
– Cyclobutadiene has never been isolated and purified. It undergoes an extremely fast Diels–Alder dimerization.
– To avoid the Diels–Alder reaction, cyclobutadiene has been prepared at low concentrations in the gas phase and as individual molecules trapped in frozen argon at low temperatures. This is not the behavior we expect from a molecule with exceptional stability!
– In 1911, Richard Willstäer synthesized cyclooctatetraene and found that it reacts like a normal polyene.
– Bromine adds readily to cyclooctatetraene, and permanganate oxidizes its double bonds.
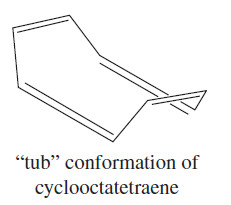
– This evidence shows that cyclooctatetraene is much less stable than benzene. In fact, structural studies have shown that cyclooctatetraene is not planar.
– It is most stable in a “tub” conformation, with poor overlap between adjacent pi bonds.
References:
- Organic chemistry / L.G. Wade, Jr / 8th ed, 2013 / Pearson Education, Inc. USA.
- Fundamental of Organic Chemistry / John McMurry, Cornell University/ 8th ed, 2016 / Cengage Learningm, Inc. USA.
- Organic Chemistry / T.W. Graham Solomons, Craig B. Fryhle , Scott A. Snyder / 11 ed, 2014/ John Wiley & Sons, Inc. USA.
- Unergraduate Organic Chemistry /Dr. Jagdamba Singh, Dr. L.D.S Yadav / 1st ed, 2010/ Pragati prakashan Educational Publishers, India.