Polynuclear Aromatic Hydrocarbons
Polynuclear Aromatic Hydrocarbons
– The polynuclear aromatic hydrocarbons (abbreviated PAHs or PNAs) are composed of two or more fused benzene rings.
– Fused rings share two carbon atoms and the bond between them.
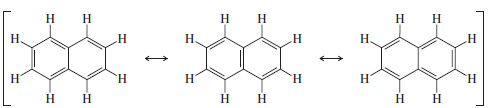
– Naphthalene is the simplest fused aromatic compound, consisting of two fused benzene rings.
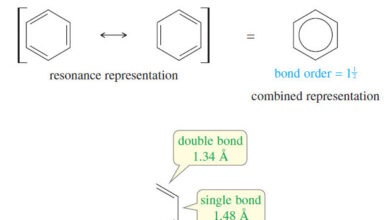
– We represent naphthalene by using one of the three Kekulé resonance structures or using the circle notation for the aromatic rings.
– The two aromatic rings in naphthalene contain a total of 10 pi electrons.
– Two isolated aromatic rings would contain 6 pi electrons in each aromatic system, for a total of 12.
– The smaller amount of electron density gives naphthalene less than twice the resonance energy of benzene: 252 kJ mol (60 kcal mol), or 126 kJ (30 kcal), per aromatic ring, compared with benzene’s resonance energy of 151 kJ mol (36 kcal mol).
– Anthracene and Phenanthrene As the number of fused aromatic rings increases, the resonance energy per ring continues to decrease and the compounds become more reactive.
– Tricyclic anthracene has a resonance energy of 351 kJ mol (84 kcal mol), or 117 kJ (28 kcal), per aromatic ring.
– Phenanthrene has a slightly higher resonance energy of 381 kJ mol (91 kcal mol), or about 127 kJ (30 kcal), per aromatic ring.
– Each of these compounds has only 14 pi electrons in its three aromatic rings, compared with 18 electrons for three separate benzene rings.
– Because they are not as strongly stabilized as benzene, anthracene and phenanthrene can undergo addition reactions that are more characteristic of their nonaromatic polyene relatives.
– Anthracene undergoes 1,4-addition at the 9- and 10-positions to give a product with two isolated, fully aromatic benzene rings.
– Similarly, phenanthrene undergoes 1,2-addition at the 9- and 10-positions to give a product with two fully aromatic rings. (Because they are less likely to be substituted, the bridgehead carbon atoms of fused aromatics are often left unnumbered.)
Larger Polynuclear Aromatic Hydrocarbons
– Larger polynuclear aromatic hydrocarbons have more fused rings than anthracene and phenanthrene, and they have less resonance energy per ring and are more reactive.
– In drawing most of these large PAHs, we have to select which Kekulé structures to use in order to make their rings appear aromatic.
– There is a high level of interest in the larger PAHs because they are formed in most combustion processes and many of them are carcinogenic (capable of causing cancer).
– The following three compounds, for example, are present in tobacco smoke.
– These compounds are so hazardous that laboratories must install special containment facilities to work with them, yet smokers expose their lung tissues to them every time they smoke a cigarette.
– Benzo[a]pyrene, one of the most thoroughly studied carcinogens, is formed whenever organic compounds undergo incomplete combustion.
– For example, benzo[a]pyrene is found in chimney soot, in broiled steaks, and in cigarette smoke.
– Long before our ancestors learned to use fire, they were exposed to benzo[a]pyrene in the smoke and ash from forest fires.
– Its carcinogenic effects appear to result from its epoxidation to arene oxides, which can be attacked by nucleophilic sites of DNA.
– The resulting DNA derivatives cannot be properly transcribed. On replication, they cause errors that produce mutations in the genes.
References:
- Organic chemistry / L.G. Wade, Jr / 8th ed, 2013 / Pearson Education, Inc. USA.
- Fundamental of Organic Chemistry / John McMurry, Cornell University/ 8th ed, 2016 / Cengage Learningm, Inc. USA.
- Organic Chemistry / T.W. Graham Solomons, Craig B. Fryhle , Scott A. Snyder / 11 ed, 2014/ John Wiley & Sons, Inc. USA.
- Unergraduate Organic Chemistry /Dr. Jagdamba Singh, Dr. L.D.S Yadav / 1st ed, 2010/ Pragati prakashan Educational Publishers, India.