-
Organic Chemistry
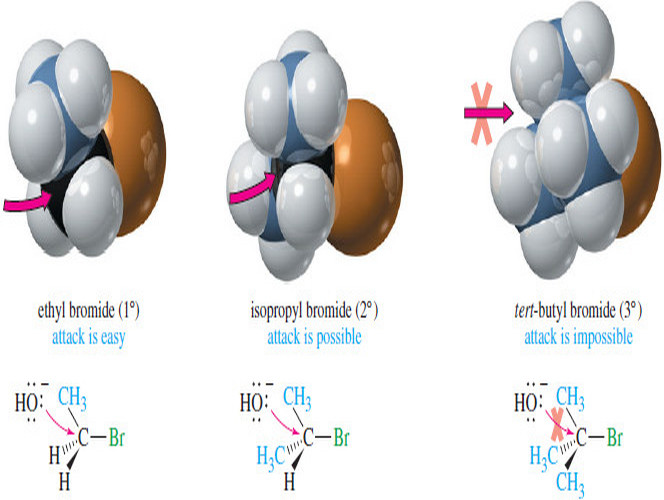
Reactivity of the Substrate in SN2 Reactions
Reactivity of the Substrate in SN2 Reactions – We will often refer to the alkyl halide as the substrate: literally,…
Read More » -
Organic Chemistry
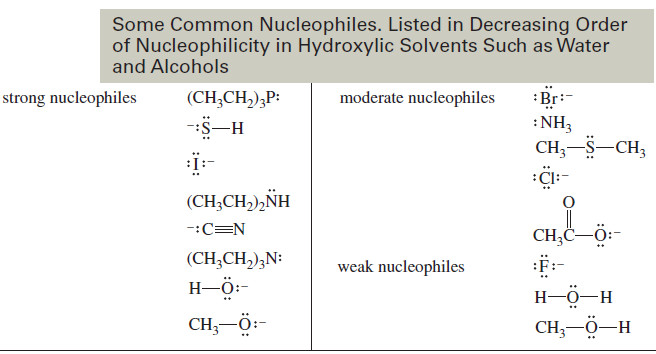
Factors Affecting SN2 Reactions: Strength of the Nucleophile
Factors Affecting SN2 Reactions: Strength of the Nucleophile – we will discuss Factors Affecting SN2 Reactions especially Strength of the…
Read More » -
Organic Chemistry
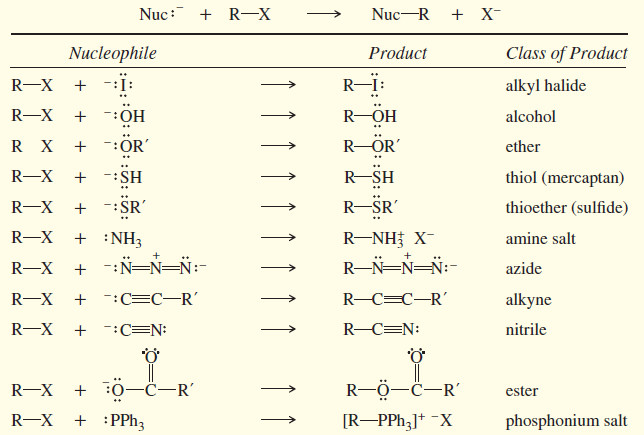
SN2 reaction of Alkyl halides
In this subject Second-Order Nucleophilic Substitution: The SN2 Reaction of Alkyl halides will be discussed Reactions of Alkyl Halides: Substitution…
Read More » -
Organic Chemistry
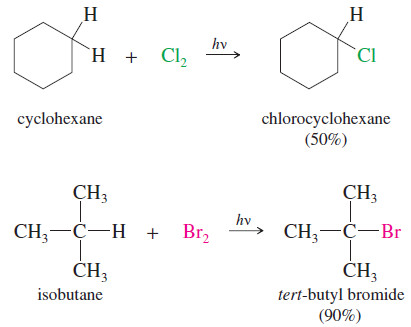
Preparation of alkyl halides
Preparation of alkyl halides – Most Methods of preparation of alkyl halides exploit the chemistry of functional groups we have…
Read More » -
Organic Chemistry
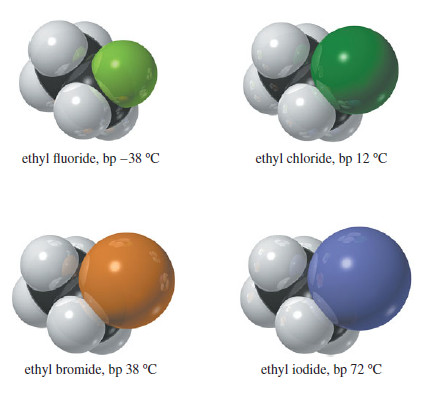
Physical Properties of Alkyl Halides
Physical Properties of Alkyl Halides will be discussed such as dipole moment, London force, Dipole–dipole attractions, densities of common alkyl…
Read More » -
Organic Chemistry
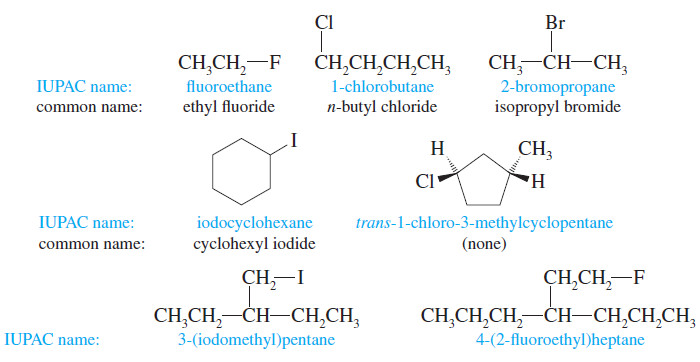
Nomenclature of Alkyl Halides
Introduction to Alkyl Halides – In this subject , we consider Nomenclature of alkyl halides. – Our study of organic…
Read More » -
Organic Chemistry
Essential terms in Stereochemistry
Essential terms in Stereochemistry stereochemistry – stereochemistry is The study of the three-dimensional structure of molecules. – It is the…
Read More » -
Organic Chemistry
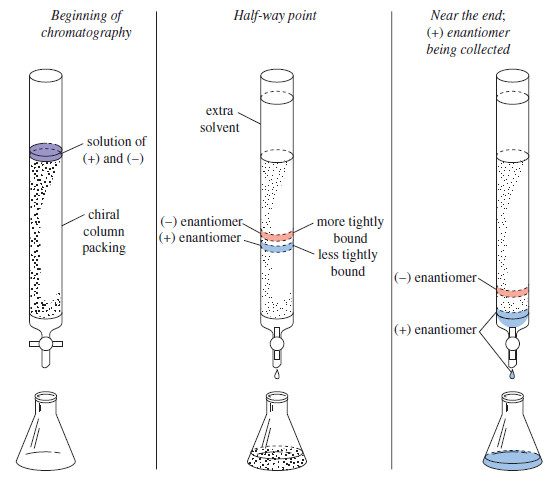
Resolution of Enantiomers
Resolution of Enantiomers – Pure enantiomers of optically active compounds are often obtained by isolation from biological sources. – Most…
Read More » -
Organic Chemistry
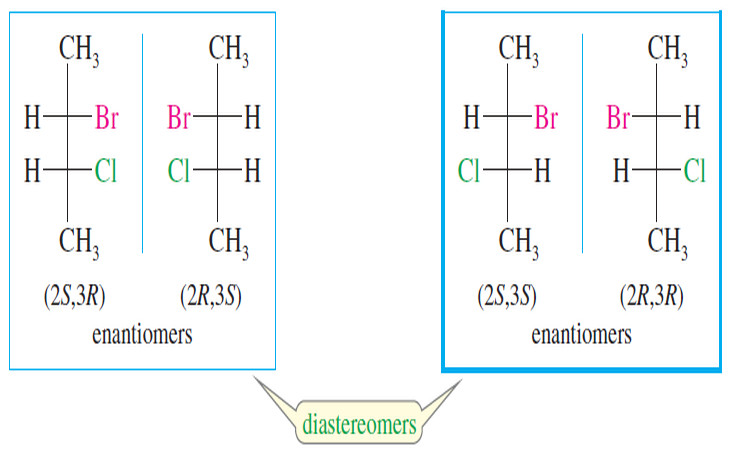
Physical Properties of Diastereomers
What is Diastereomers? – We have defined stereoisomers as isomers whose atoms are bonded together in the same order but…
Read More » -
Organic Chemistry
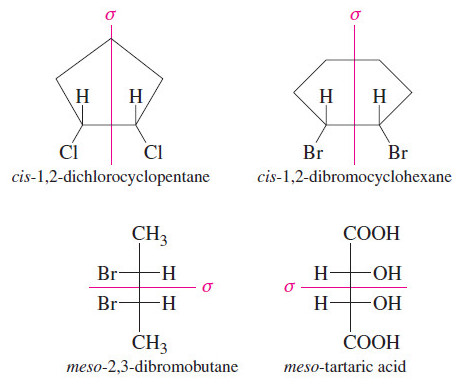
Meso Compounds
Meso Compounds – Compounds that are achiral even though they have asymmetric carbon atoms are called meso compounds. – The…
Read More » -
Organic Chemistry
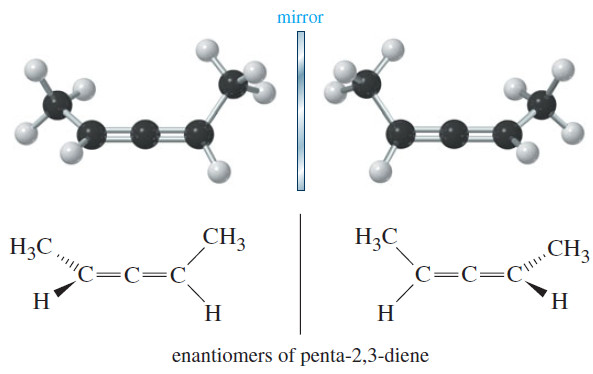
Chiral Compounds without Asymmetric Atom
Chiral Compounds without Asymmetric Atoms – Most chiral organic compounds have at least one asymmetric carbon atom. – Some compounds…
Read More » -
Organic Chemistry
What is Diastereomers?
Diastereomers – We have defined stereoisomers as isomers whose atoms are bonded together in the same order but differ in…
Read More » -
Organic Chemistry
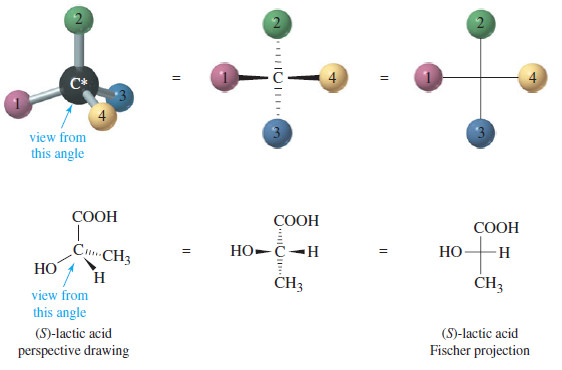
Drawing Fischer Projections
In this subject we will discuss How to draw Fischer projections Introduction to Fischer Projections – We have been using…
Read More » -
Organic Chemistry
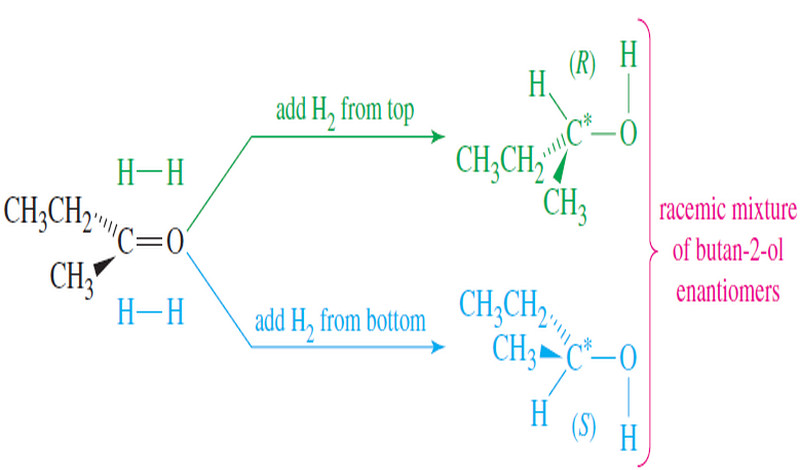
Racemic Mixtures
Racemic Mixtures – Suppose we had a mixture of equal amounts of (+)-butan-2-ol and (-)-butan-2-ol – The (+) isomer would…
Read More » -
Organic Chemistry
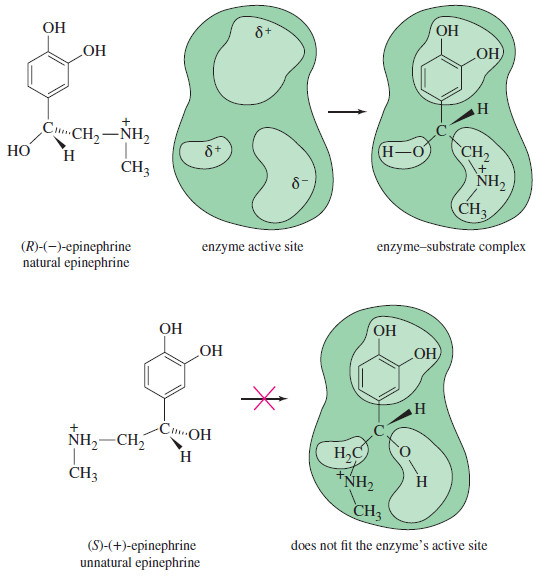
Biological Discrimination of Enantiomers
Biological Discrimination of Enantiomers – If the direction of rotation of polarized light were the only difference between enantiomers, one…
Read More » -
Organic Chemistry
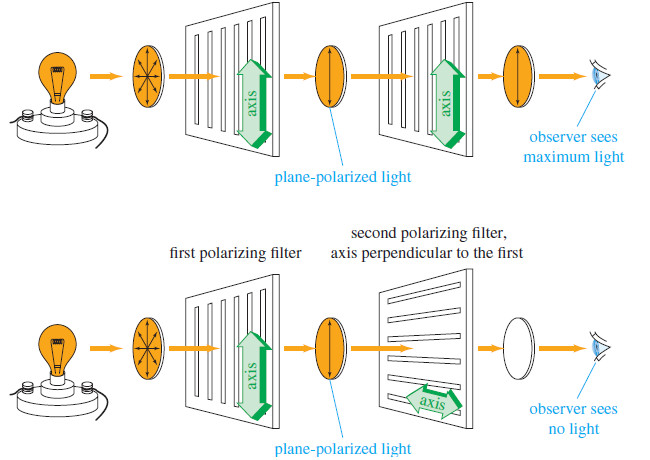
Optical Activity in Organic Compounds
– Rotation of the plane of polarized light is called optical activity, and substances that rotate the plane of polarized…
Read More » -
Organic Chemistry
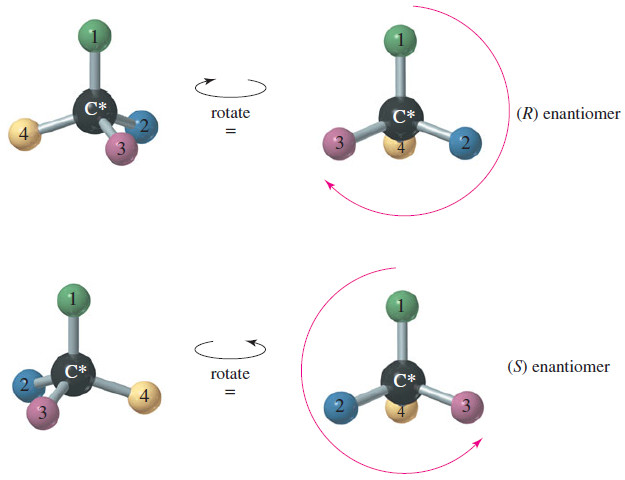
(R) and (S) of Asymmetric Carbon Atoms
(R) and (S) Nomenclature of Asymmetric Carbon Atoms – Alanine is one of the amino acids found in common proteins.…
Read More » -
Organic Chemistry
Chirality in Organic Chemistry
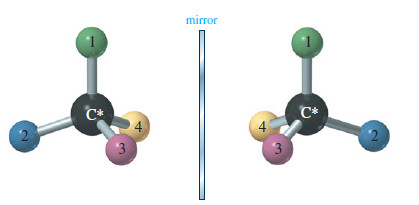
What is Chirality? – What is the difference between your left hand and your right hand? They look similar, yet…
Read More » -
Physical Chemistry
Laws of Osmotic Pressure
Laws of Osmotic Pressure – From a study of the experimental results obtained by Pfeffer, van’t Hoff showed that for…
Read More » -
Physical Chemistry
Theories of Osmosis
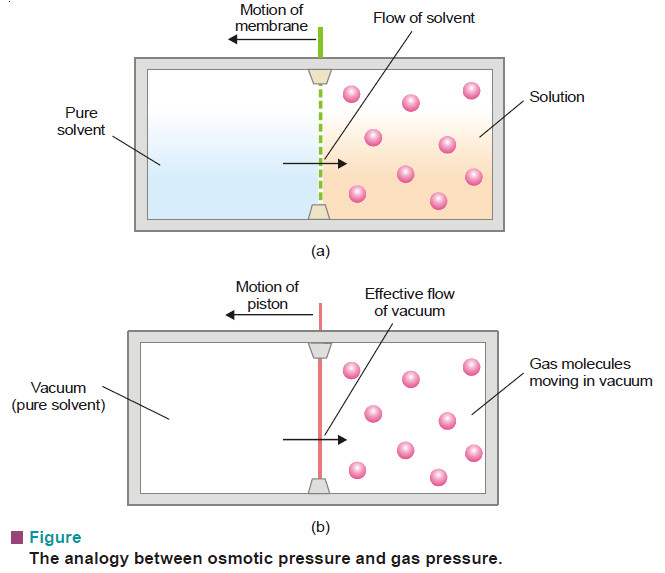
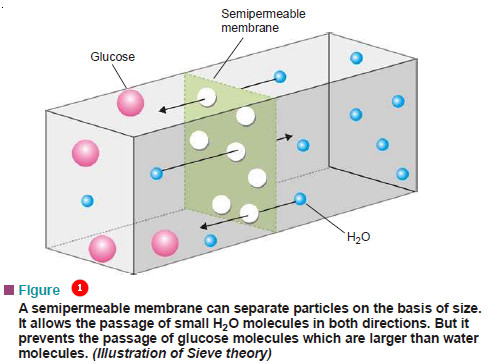
Theories of Osmosis – Here we will discuss some theories of osmosis. – Several theories have been advanced to explain…
Read More »