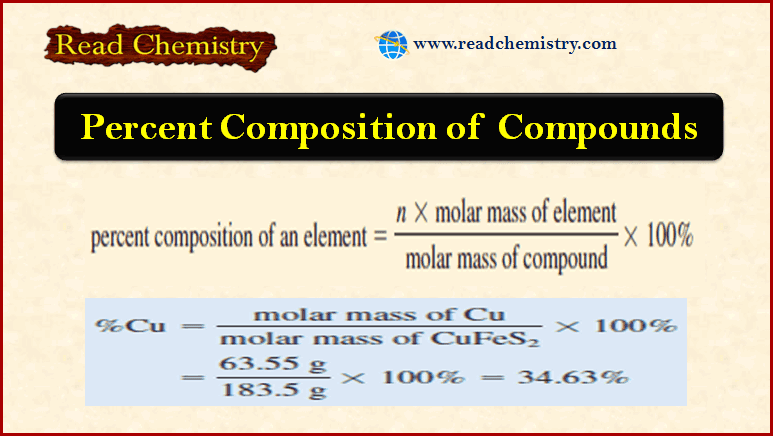
Applications of Gravimetric methods
Applications of Gravimetric methods
– Gravimetric methods have been developed for most inorganic anions and cations, as well as for such neutral species as water, sulfur dioxide, carbon dioxide, and iodine.
– A variety of organic substances can also be determined gravimetrically.
– Examples include lactose in milk products, salicylates in drug preparations, phenolphthalein in laxatives, nicotine in pesticides, cholesterol in cereals, and benzaldehyde in almond extracts.
– Indeed, gravimetric methods are among the most widely applicable of all analytical procedures.
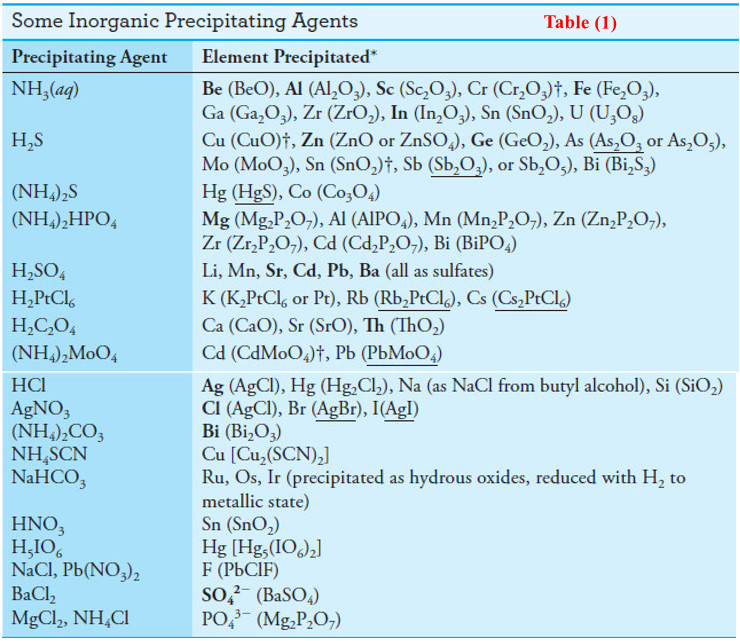
(1) Inorganic Precipitating Agents
– Table (1) lists common inorganic precipitating agents.
– These reagents typically form slightly soluble salts or hydrous oxides with the analyte.
– As you can see from the many entries for each reagent, few inorganic reagents are selective.
* Boldface type indicates that gravimetric analysis is the preferred method for the element or ion.
The weighed form is indicated in parentheses.
† A dagger indicates that the gravimetric methods are seldom used. An underscore indicates the most reliable gravimetric method.
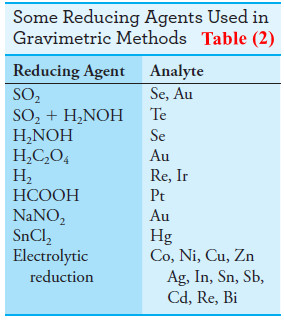
(2) Reducing Agents
– Table (2) lists several reagents that convert an analyte to its elemental form for weighing.
(3) Organic Precipitating Agents
– Numerous organic reagents have been developed for the gravimetric determination of inorganic species.
– Some of these reagents are significantly more selective in their reactions than are most of the inorganic reagents listed in Table 12-2.
– There are two types of organic reagents: one forms slightly soluble nonionic products called coordination compounds, and the other forms products in which the bonding between the inorganic species and the reagent is largely ionic.
– Organic reagents that yield sparingly soluble coordination compounds typically contain at least two functional groups.
– Each of these groups is capable of bonding with a cation by donating a pair of electrons.
– The functional groups are located in the molecule such that a five- or six-membered ring results from the reaction.
– Reagents that form compounds of this type are called chelating agents, and their products are called chelates.
– Metal chelates are relatively nonpolar and, as a consequence, have solubilities that are low in water but high in organic liquids.
– Usually, these compounds possess low densities and are often intensely colored.
– Because they are not wetted by water, coordination compounds are easily freed of moisture at low temperatures.
– Two widely used chelating reagents are described in the paragraphs that follow.
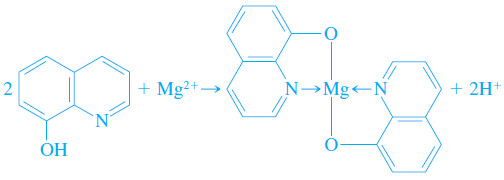
8-Hydroxyquinoline (oxine)
– Approximately two dozen cations form sparingly soluble chelates with 8-hydroxyquinoline.
– The structure of magnesium 8-hydroxyquinolate is typical of these chelates.
– The solubilities of metal 8-hydroxyquinolates vary widely from cation to cation and are pH dependent because 8-hydroxyquinoline is always deprotonated during a chelation reaction.
– Therefore, we can achieve a considerable degree of selectivity in the use of 8-hydroxyquinoline by controlling pH.
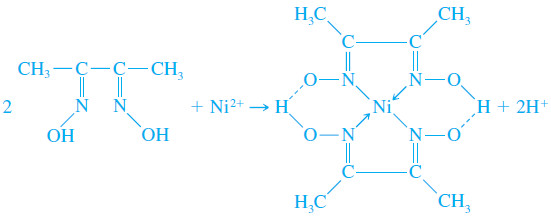
Dimethylglyoxime
– Dimethylglyoxime is an organic precipitating agent of unparalleled specificity.
– Only nickel(II) is precipitated from a weakly alkaline solution. The reaction is:
– This precipitate is so bulky that only small amounts of nickel can be handled conveniently.
– It also has an exasperating tendency to creep up the sides of the container as it is filtered and washed.
– The solid is conveniently dried at 110°C and has the composition C8H14N4NiO4.
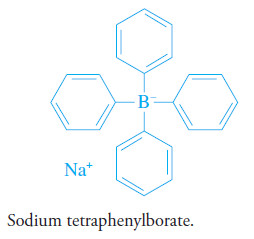
Sodium Tetraphenylborate
– Sodium tetraphenylborate, (C6H5)4B2Na+, is an important example of an organic precipitating reagent that forms salt-like precipitates.
– In cold mineral acid solutions, it is a near-specific precipitating agent for potassium and ammonium ions.
– The precipitates have stoichiometric composition and contain one mole of potassium or ammonium ion for each mole of tetraphenylborate ion.
– These ionic compounds are easily filtered and can be brought to constant mass at 105°C to 120°C.
– Only mercury(II), rubidium, and cesium interfere and must be removed by prior treatment.
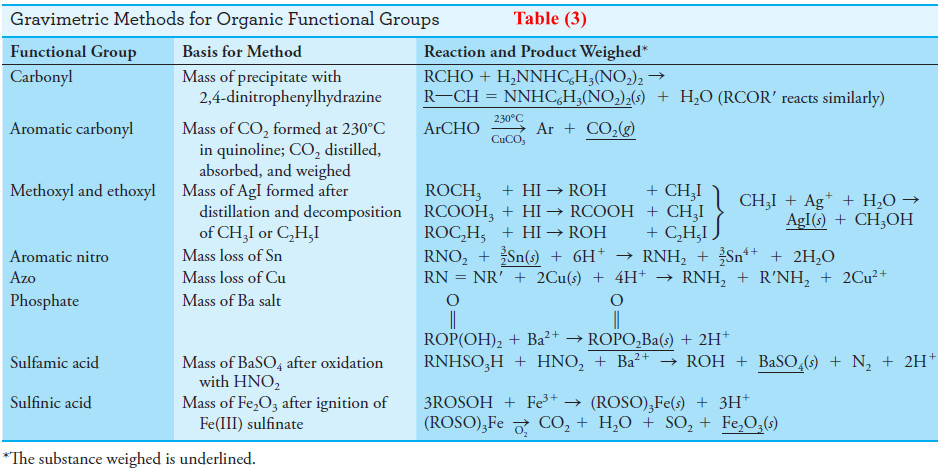
(4) Organic Functional Group Analysis
– Several reagents react selectively with certain organic functional groups and thus can be used for the determination of most compounds containing these groups.
– A list of gravimetric functional group reagents is given in Table (3).
– Many of the reactions shown can also be used for volumetric and spectrophotometric determinations.
(5) Volatilization Gravimetry
– The two most common gravimetric methods based on volatilization are those for determining water and carbon dioxide.
– Water is quantitatively distilled from many materials by heating.
– In direct determination, water vapor is collected on any of several solid desiccants, and its mass is determined from the mass gain of the desiccant.
– The indirect method in which the amount of water is determined by the loss of mass of the sample during heating is less satisfactory because it must be assumed that water is the only component that is volatilized.
– This assumption can present problems, however, if any component of the precipitate is volatile.
– Nevertheless, the indirect method is widely used to determine water in items of commerce. For example, a semiautomated instrument for the determination of moisture in cereal grains can be purchased.
– It consists of a platform balance on which a 10-g sample is heated with an infrared lamp. The percent moisture is read directly.
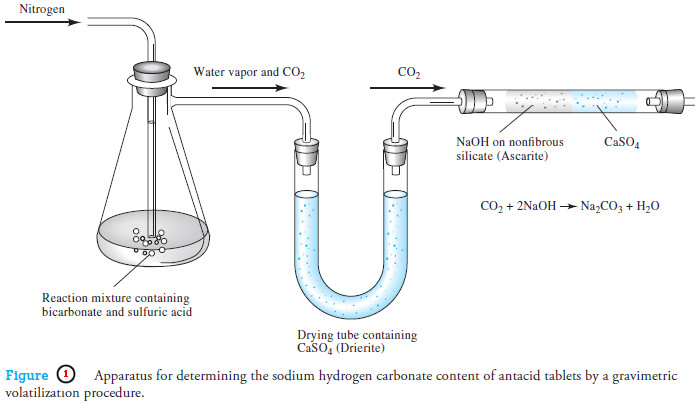
– An example of a gravimetric procedure involving volatilization of carbon dioxide is the determination of the sodium hydrogen carbonate content of antacid tablets.
– A weighed sample of the finely ground tablets is treated with dilute sulfuric acid to convert the sodium hydrogen carbonate to carbon dioxide:
NaHCO3 (aq) + H2SO4 (aq) → CO2 (g) + H2O (l) + NaHSO4 (aq)
– As shown in Figure (1), this reaction is carried out in a flask connected first to a tube containing CaSO4 that removes water vapor from the initial reaction stream to produce a stream of pure CO2 in nitrogen.
– These gases then pass through a weighed absorption tube containing the absorbent Ascarite II,8 which consists of sodium hydroxide absorbed on a nonfibrous silicate.
– This material retains carbon dioxide by the reaction:
2NaOH + CO2 → Na2CO3 + H2O
– The absorption tube must also contain a desiccant such as CaSO4 to prevent loss of the water produced by this last reaction.
– Sulfides and sulfites can also be determined by volatilization.
– Hydrogen sulfide or sulfur dioxide evolved from the sample after treatment with acid is collected in a suitable absorbent.
– Finally, the classical method for the determination of carbon and hydrogen in organic compounds is a gravimetric volatilization procedure in which the combustion products (H2O and CO2) are collected selectively on weighed absorbents.
– The increase in mass serves as the analytical variable.
Reference
- Modern analytical chemistry / David Harvey / The McGraw-Hill Companies, Inc./ , 2000 . USA
- Dean’s Analytical Chemistry Handbook / Pradyot Patnaik / The McGraw-Hill Companies, 2nd Editionm, 2004 .USA
- Fundamentals of analytical chemistry / Douglas A. Skoog, Donald M. West, F. James Holler, Stanley R. Crouch. (ninth edition) , 2014 . USA