Analytical Chemistry involves techniques and methods to identify, quantify, and understand chemical substances. It supports quality control, research, environmental analysis, and forensic science, ensuring accuracy and precision in measurements.
Analytical Chemistry
-
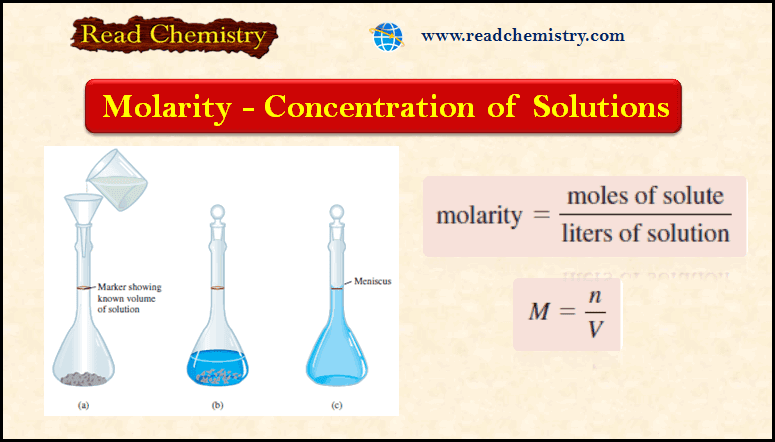
Molarity: definition, formula, solved Problems
– In this subject, we will discuss the Molarity: definition, formula, solved Problems – To study solution stoichiometry, we must…
Read More » -
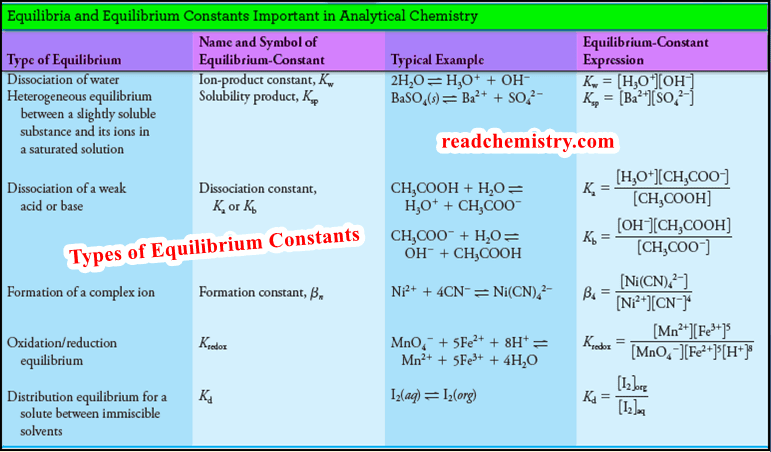
Types of Equilibrium Constants used in Analytical Chemistry
– In this subject, we will discuss Types of Equilibrium Constants used in Analytical Chemistry. Chemical Equilibrium and Equilibrium constants…
Read More » -
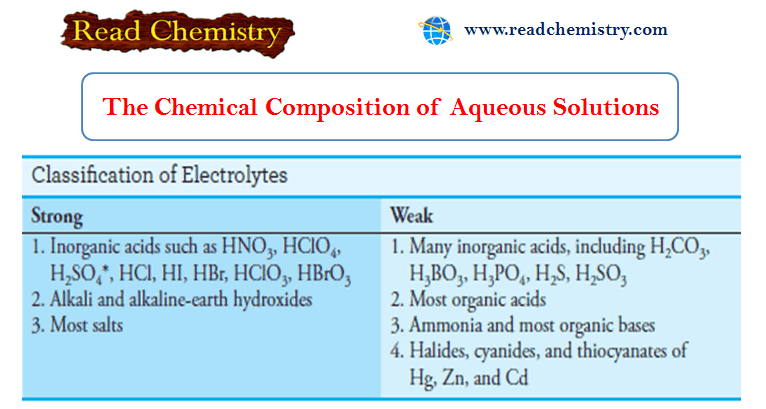
The Chemical Composition of Aqueous Solution
– In this subject, we will discuss the Chemical Composition of Aqueous Solution – Water is the most plentiful solvent…
Read More » -
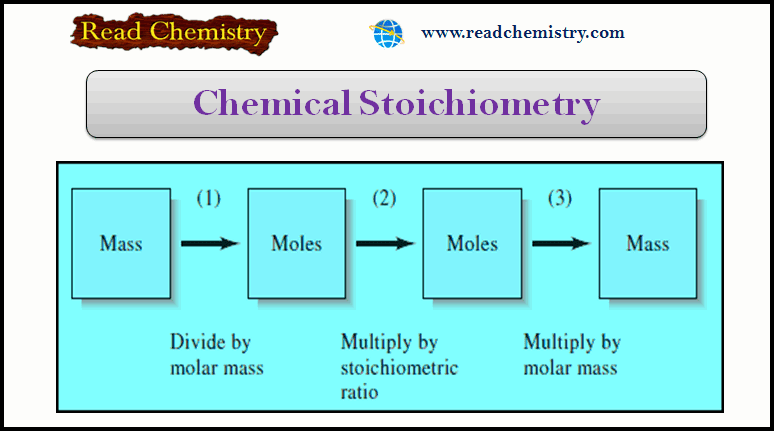
Chemical Stoichiometry: Definition, Formula, Examples
In this subject, we will discuss the Chemical Stoichiometry: Definition, Formula, Example Chemical Stoichiometry – Stoichiometry is the quantitative relationship…
Read More » -
Concentration of Solutions: Definitions, Formulas, Solved Problems
– In this subject, we will discuss the Concentration of Solutions Concentration of Solutions (Definitions, Formulas, Solved Problems). Concentration of…
Read More » -
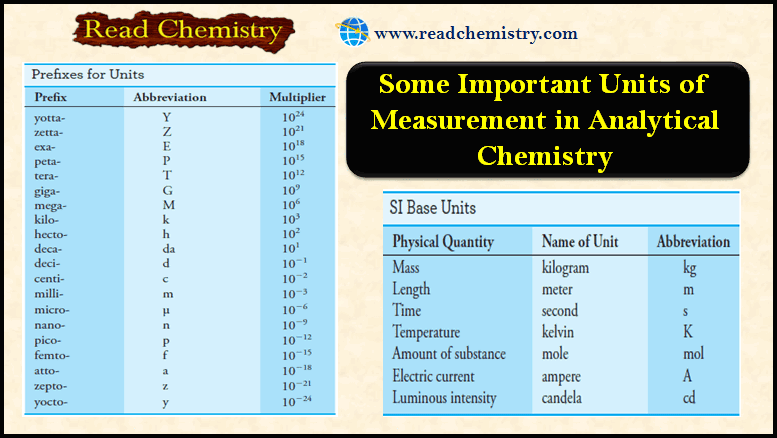
Some Important Units of Measurement in Analytical Chemistry
SI Units – Scientists throughout the world have adopted a standardized system of units known as the International…
Read More » -
Safety in the laboratory
– In this subject, we will discuss the Safety in the laboratory Safety in The Laboratory – There is necessarily…
Read More » -
The Laboratory Notebook
The Laboratory Notebook – A laboratory notebook is needed to record measurements and observations concerning an analysis. – The book…
Read More » -
Calibration of Volumetric Glassware in the laboratory
– In this subject, we will discuss How to Calibrate Volumetric Glassware in the laboratory Calibration of Volumetric Glassware –…
Read More » -
Volumetric Flask: Overview, Uses, Function
Volumetric Flask – A volumetric flask is manufactured with capacities ranging from 5 mL to 5 L and is…
Read More » -
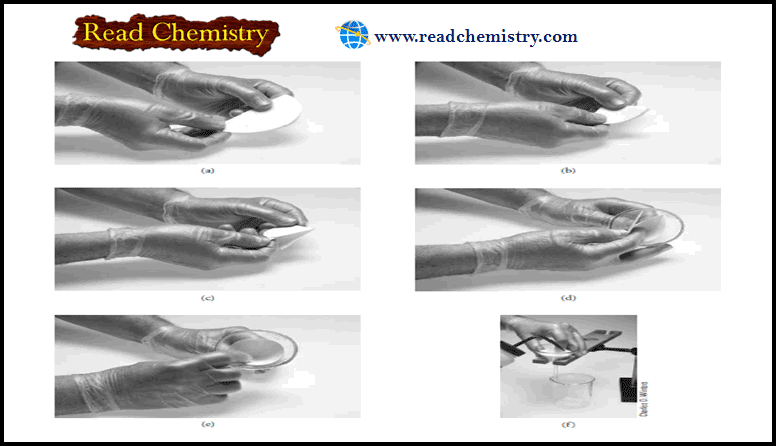
Pipets : Overview, Uses, Function, Cleaning
Pipets – Pipets permit the transfer of accurately known volumes from one container to another. – Common types are shown…
Read More » -
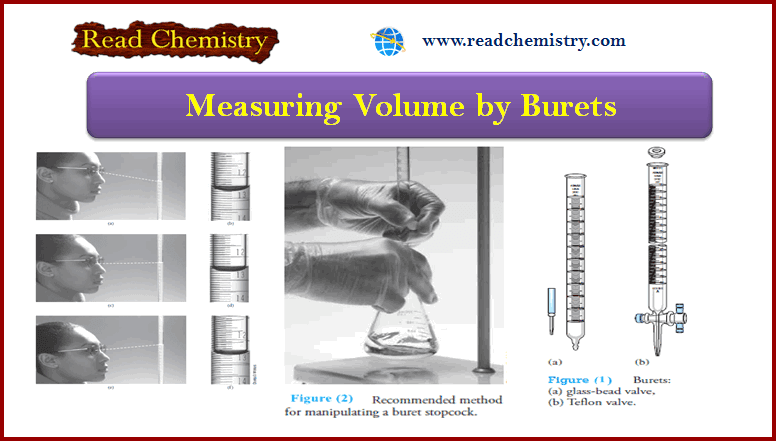
Burets : Overview, Uses, Function, Cleaning
Burets – Burets, like measuring pipets, make it possible to deliver any volume up to the maximum capacity of the…
Read More » -
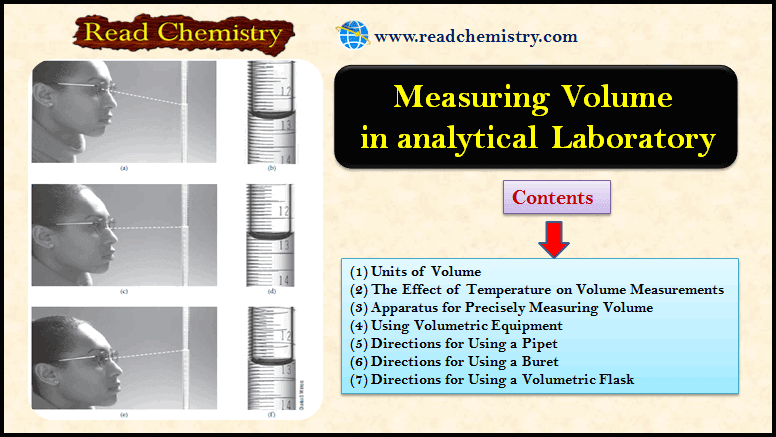
Measuring Volume by Pipets, Burets, Volumetric Flask
– The precise measurement of volume is as important to many analytical methods as the precise measurement of mass. (1)…
Read More » -
Filtration and Ignition of Solids
– In this subject, we will discuss the Filtration and Ignition of Solids. – Several techniques and experimental arrangements allow…
Read More » -
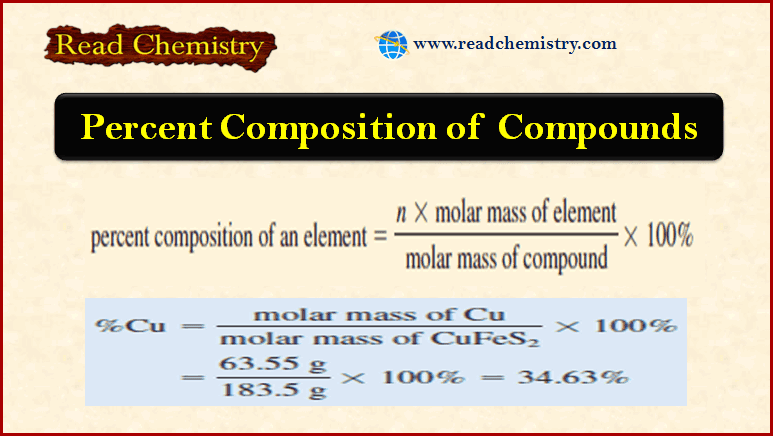
Percent Composition of Compounds
Percent Composition of Compounds **As we have seen, the formula of a compound tells us the numbers of atoms…
Read More » -
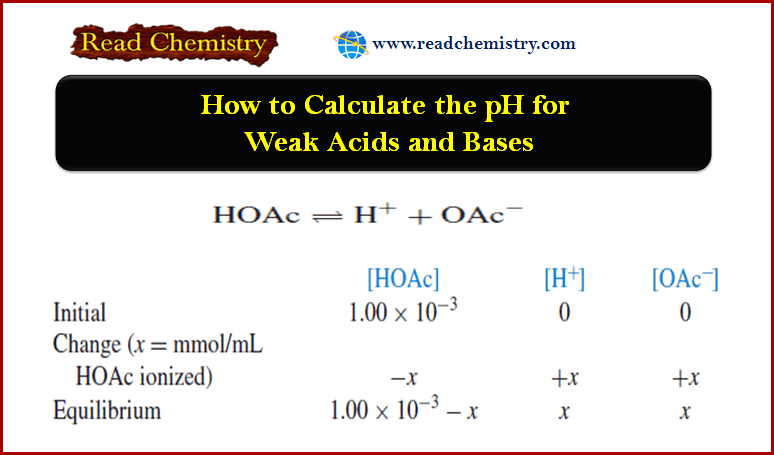
Calculating the pH of Weak Acid and Base Solutions
– In this subject, we will discuss Calculating the pH of Weak Acid and Base Solutions Total and partial ionization…
Read More » -
Analytical Chemistry book by Gary D.Christian – Free Download
– In this subject, we will discuss the Free Download Analytical Chemistry book (7th edition) by Gary D.Christian Introduction to…
Read More » -
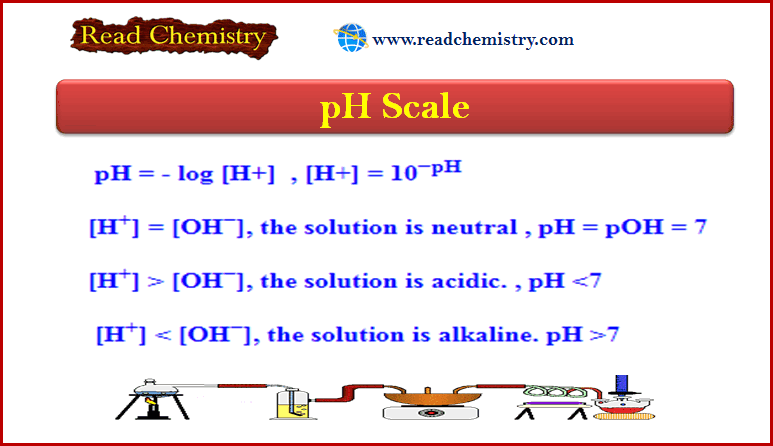
pH Scale: Definition, formula, Notes, Solved problems
– In this subject, we will discuss the pH Scale: Definition, formula, Notes, Solved problems pH Scale – The concentration…
Read More » -
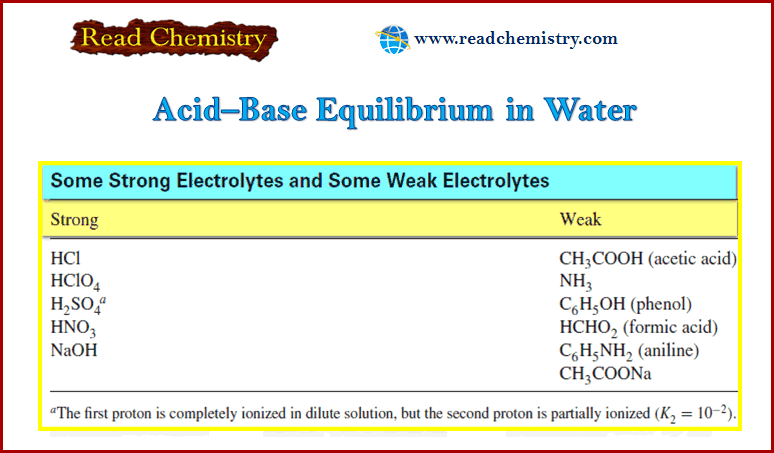
Acid-Base Equilibrium in Water
– In this subject, we will discuss Acid-Base Equilibrium in Water. Acid-Base Equilibrium in Water – When an acid or…
Read More » -
Acid-Base Theories: Arrhenius, Lewis, and Bronsted-Lowry Theory
– In this subject, we will discuss Acid-Base Theories: Arrhenius, Lewis, and Bronsted-Lowry Theory Acid-base Theories – Several acid–base theories…
Read More »