Organic Chemistry
Organic Chemistry focuses on the structure, properties, and reactions of carbon-containing compounds. It’s essential in pharmaceuticals, polymers, and biochemistry, exploring mechanisms, functional groups, and synthesis of complex molecules.
-
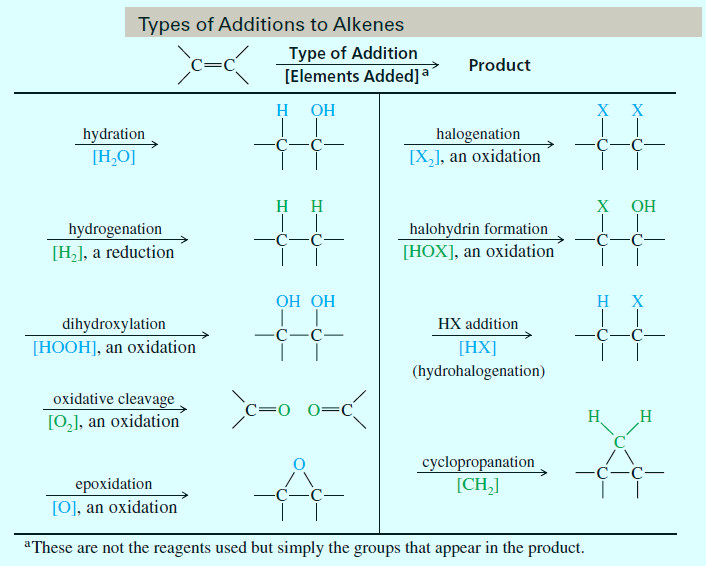
Electrophilic Addition to Alkenes
Electrophilic Addition to Alkenes Reactivity of the Carbon–Carbon Double Bond – All alkenes have a common feature: a carbon–carbon double…
Read More » -
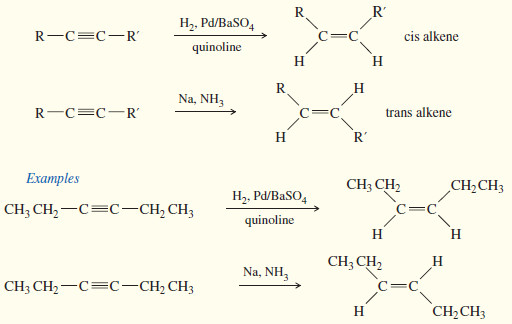
Synthesis of Alkenes – Six methods
Methods for Synthesis of Alkenes – Six methods for Synthesis of Alkenes will be discussed as follow: (1) Dehydrohalogenation of…
Read More » -
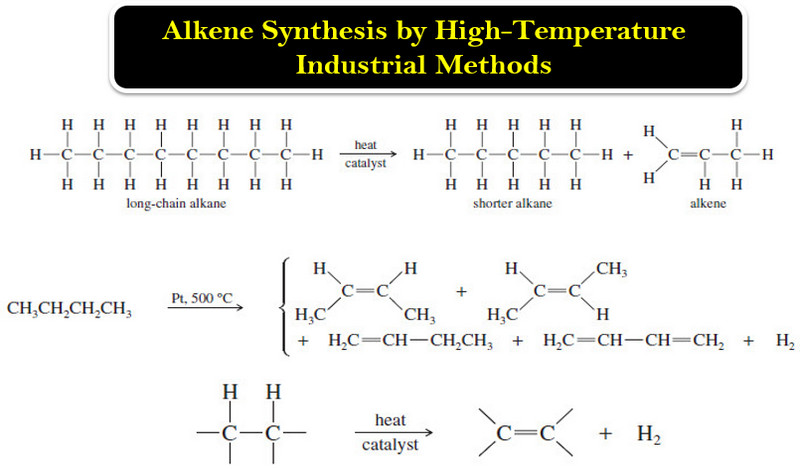
Alkene Synthesis by High-Temperature Industrial Methods
Alkene Synthesis by High-Temperature Industrial Methods (1) Catalytic Cracking of Alkanes – The least expensive way to make alkenes on…
Read More » -
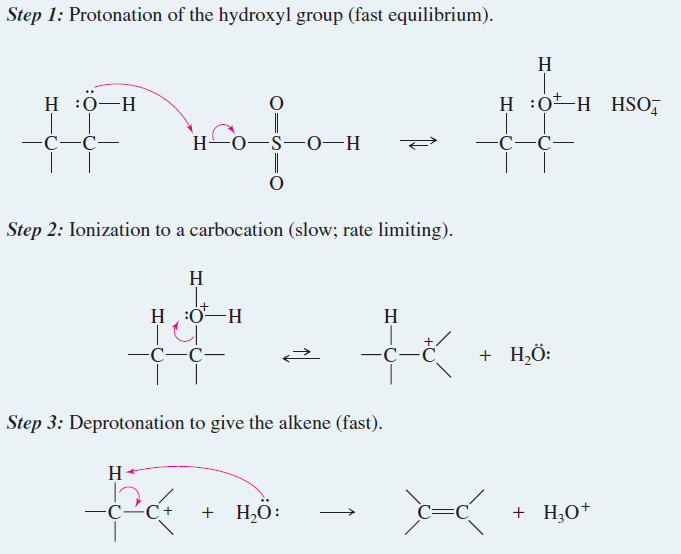
Alkene Synthesis by Dehydration of Alcohols
Alkene Synthesis by Dehydration of Alcohols – Dehydration of alcohols is a common method for making alkenes. The word dehydration…
Read More » -
Alkene Synthesis by Elimination of Alkyl Halides
Alkene Synthesis by Elimination of Alkyl Halides – Dehydrohalogenation is the elimination of a hydrogen and a halogen from an…
Read More » -
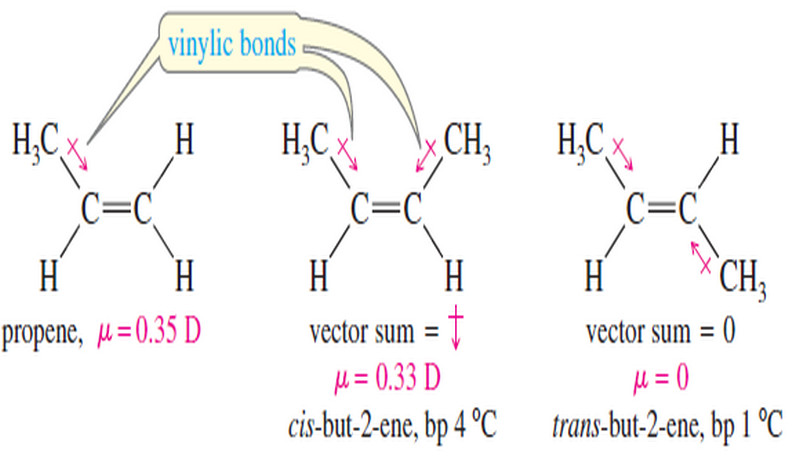
Physical Properties of Alkenes
Physical Properties of Alkenes (1) Boiling Points and Densities – Most physical properties of alkenes are similar to those of…
Read More » -
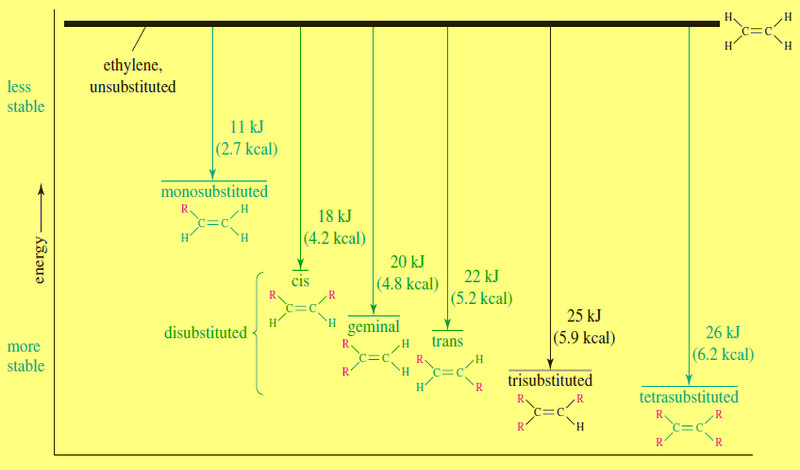
Stability of Alkenes
Stability of Alkenes – In making alkenes, we often find that the major product is the most stable alkene. –…
Read More » -
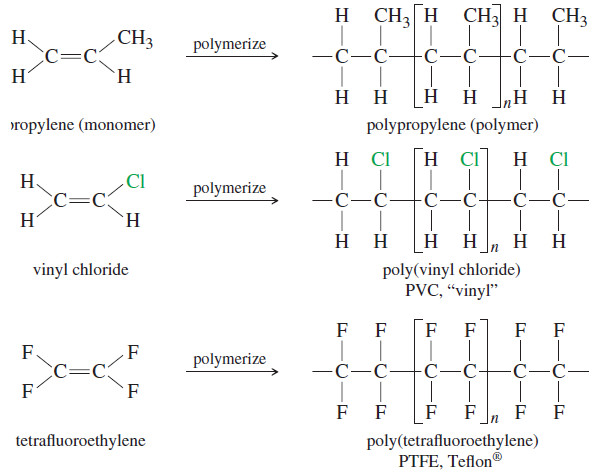
Commercial Importance of Alkenes
Commercial Importance of Alkenes – Because the carbon–carbon double bond is readily converted to other functional groups, alkenes are important…
Read More » -
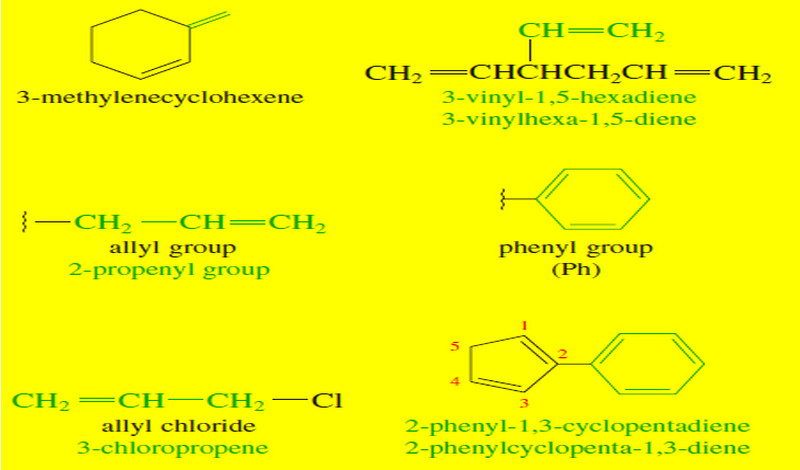
Nomenclature of Alkenes
Nomenclature of Alkenes – Simple alkenes are named much like alkanes, using the root name of the longest chain containing…
Read More » -
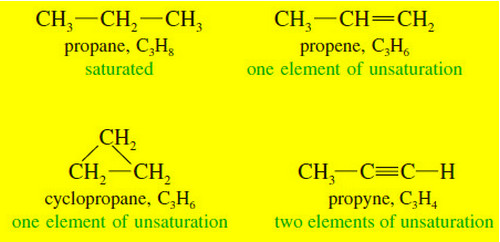
Elements of Unsaturation
Elements of Unsaturation (1) Elements of Unsaturation in Hydrocarbons – Alkenes are said to be unsaturated because they are capable…
Read More » -
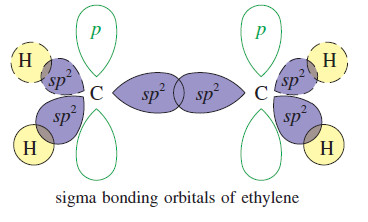
The Orbital Description of the Alkene Double Bond
The Orbital Description of the Alkene Double Bond – In a Lewis structure, the double bond of an alkene is…
Read More » -
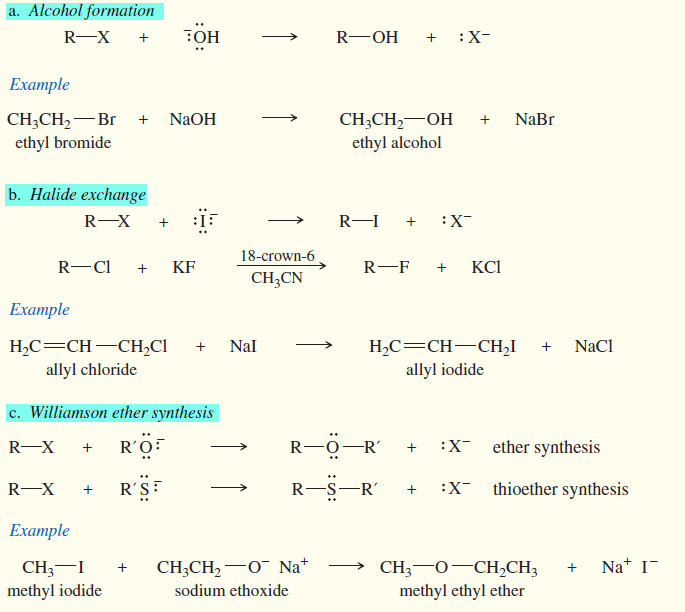
Reactions of Alkyl Halides
In this subject we will discuss the Reactions of Alkyl Halides with chemical equations and examples Introduction to Alkyl Halides…
Read More » -
Predicting SN1 SN2 E1 E2 reactions
Predicting the mechanisms: SN1, SN2, E1, E2 reactions – In this subject we will learn how to predict the the…
Read More » -
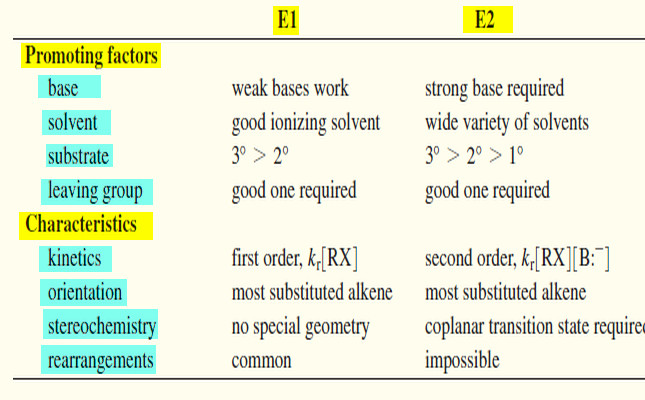
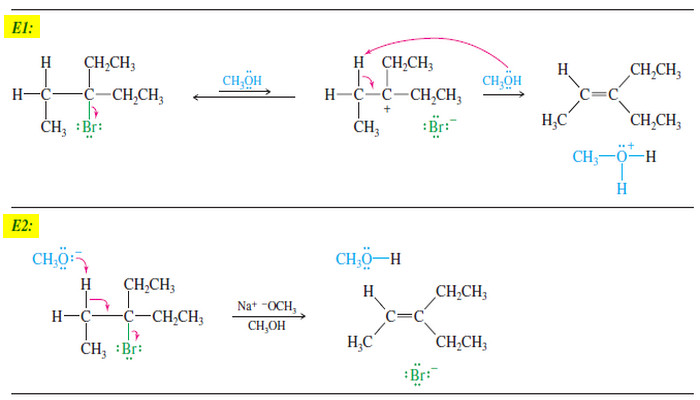
Comparison of E1 and E2 reactions
Comparison of E1 and E2 Elimination Mechanisms – Let’s summarize the major points to remember about the E1 and E2…
Read More » -
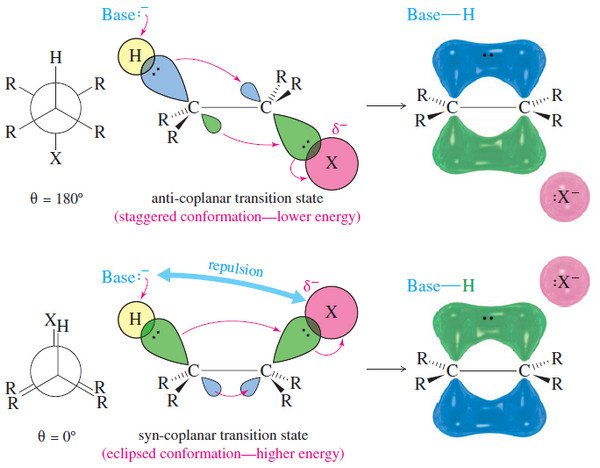
Stereochemistry of the E2 Reaction
Stereochemistry of the E2 Reaction – In this subject Stereochemistry of the E2 Reaction will be discussed – Like the…
Read More » -
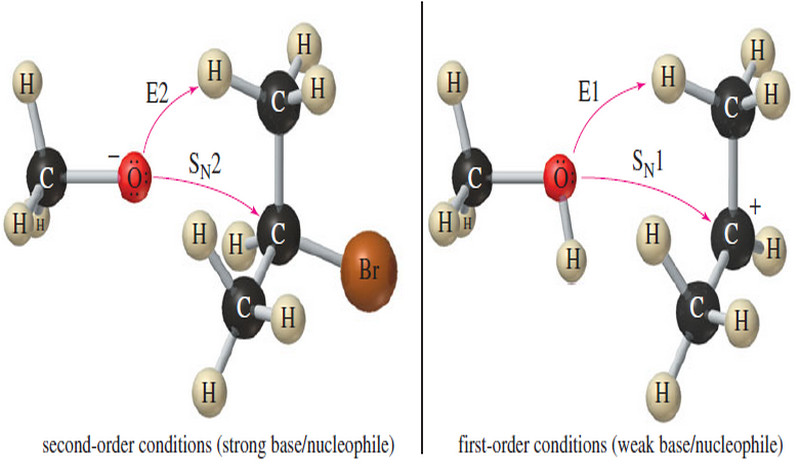
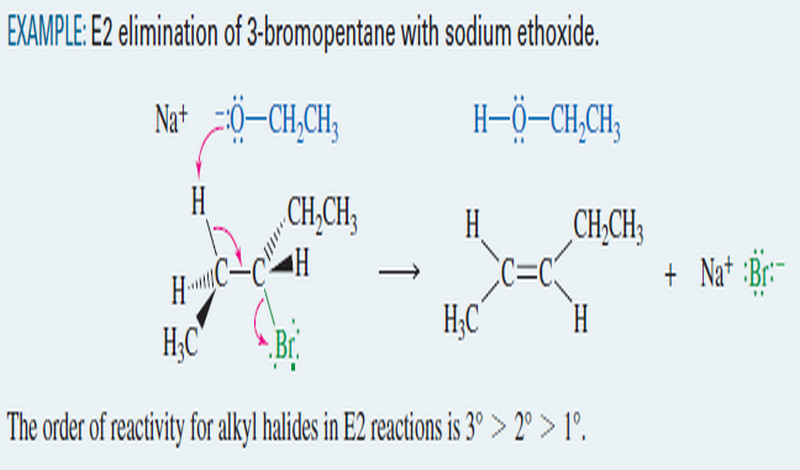
E2 Reaction : Second-Order Elimination
Second-Order Elimination: The E2 Reaction – Eliminations can also take place under second-order conditions with a strong base present. –…
Read More » -
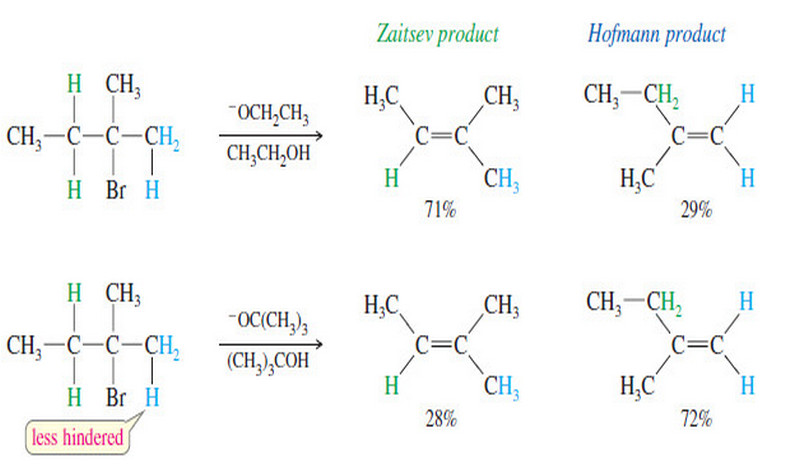
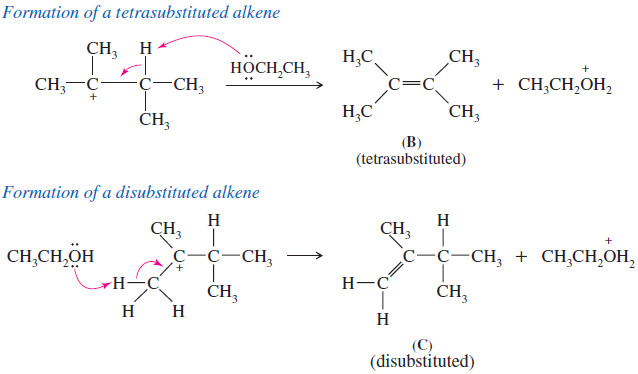
Zaitsev’s Rule : Positional Orientation of Elimination
Positional Orientation of Elimination: Zaitsev’s Rule – In this subject , Positional Orientation of Elimination: Zaitsev’s Rule will be discussed…
Read More » -
E1 Reaction : First-Order Elimination
First-Order Elimination : The E1 Reaction – An elimination involves the loss of two atoms or groups from the substrate,…
Read More » -
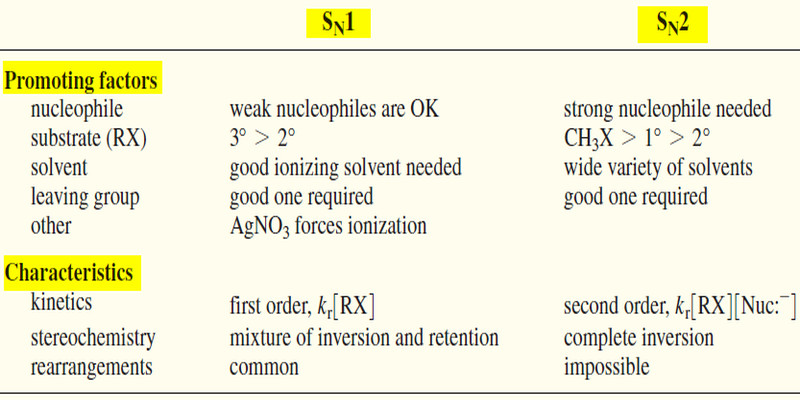
Comparison of SN1 and SN2 Reactions
Comparison of SN1 and SN2 Reactions Let’s compare what we know about the SN1 and SN2 Reactions and reactions, the…
Read More » -
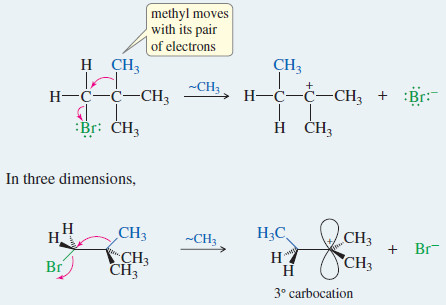
Rearrangements in the SN1 Reactions
Rearrangements in the SN1 Reactions – Carbocations frequently undergo structural changes, called rearrangements, to form more stable ions. – A…
Read More »