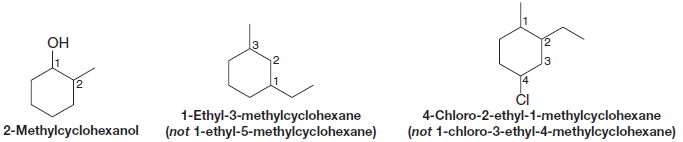Nomenclature of Cycloalkanes: Monocyclic, Bicyclic
– In this subject, we will discuss the Nomenclature of Cycloalkanes: Monocyclic, Bicyclic
What are Cycloalkanes?
– Saturated cyclic hydrocarbons are called cycloalkanes, or alicyclic (aliphatic cyclic) compounds, and have the general formula (CH2)n or CnH2n.
– Cycloalkanes are named by adding “cyclo” before the parent name of the alkane.
– Substituted cycloalkanes are named by rules similar to those for open-chain alkanes
Nomenclature of Cycloalkanes
Nomenclature of Monocyclic Cycloalkanes
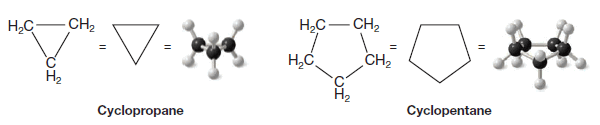
(1) Cycloalkanes with one ring and no substituents
– Count the number of carbon atoms in the ring, then add (cyclo) to the beginning of the name of the alkane with that number of carbons.
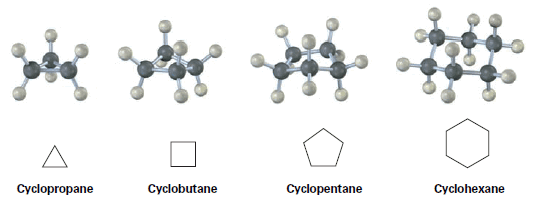
– For example, cyclopropane has three carbons and cyclopentane has five carbons.
(2) Cycloalkanes with one ring and one substituent
– Add the name of the substituent to the beginning of the parent name.
– For example, cyclohexane with an attached isopropyl group is isopropylcyclohexane.
– For compounds with only one substituent, it is not necessary to specify a number (locant) for the carbon bearing the substituent.
(3) Cycloalkanes with one ring and two or more substituents
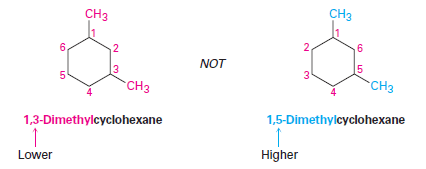
– For a ring with two substituents, begin by numbering the carbons in the ring, starting at the carbon with the substituent that is first in the alphabet and number in the direction that gives the next substituent the lowest number possible.
– When there are three or more substituents, begin at the substituent that leads to the lowest set of numbers (locants).
– The substituents are listed in alphabetical order, not according to the number of their carbon atom.
(4) When a single-ring system is attached to a single chain
– When a single ring system is attached to a single chain: with a greater number of carbon atoms, or when more than one ring system is attached to a single chain, then it is appropriate to name the compounds as cycloalkylalkanes.
(5) When two or more different alkyl groups are present
– When two or more different alkyl groups are present that could potentially take the same numbers, number them by alphabetical priority, ignoring numerical prefixes such as di- and tri-.
(6) If halogens are present
– If halogens are present: treat them just like alkyl groups.
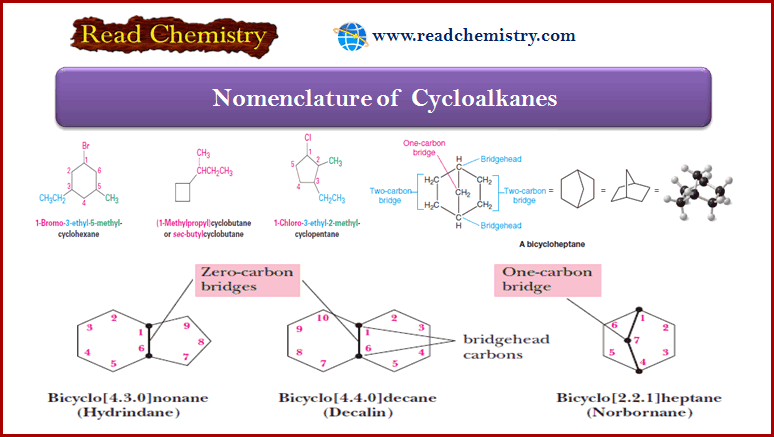
Nomenclature of Bicyclic Cycloalkanes
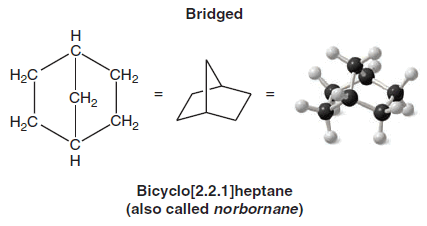
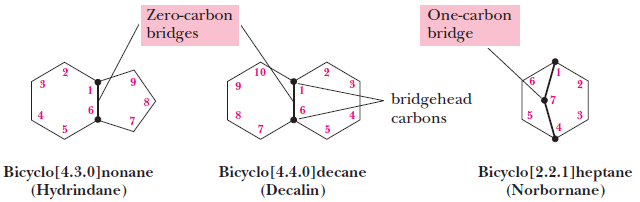
(1) We name compounds containing two fused or bridged rings as bicycloalkanes and we use the name of the alkane corresponding to the total number of carbon atoms in the rings as the parent name.
– The following compound, for example, contains seven carbon atoms and is, therefore, a bicycloheptane.
– The carbon atoms common to both rings are called bridgeheads, and each bond, or each chain of atoms connecting the bridgehead atoms, is called a bridge.
(2) We then interpose an expression in brackets within the name that denotes the number of carbon atoms in each bridge (in order of decreasing length).
– Fused rings have zero carbons in their bridge.
(3) In bicycloalkanes with substituents, we number the bridged ring system beginning at one bridgehead, proceeding first along the longest bridge to the other bridgehead, then along the next longest bridge back to the first bridgehead; the shortest bridge is numbered last.

Examples of Nomenclature of Cycloalkane
Reference:
– Organic chemistry / William H. Brown, Christopher S. Foote, Brent L. Iverson, Eric V. Anslyn, Bruce M. Novak / ( sixth edition) / 2012 / United States.
– Fundamental of Organic chemistry / John McMurry. / ( seven editions) / 2011 / United States.
– Organic chemistry / John McMurry. / ( ninth edition) / 2016 / United States.
– Organic chemistry / T.W. Graham Solomons, Craig B. Fryhle, Scott A. Snyder / ( eleventh edition) / 2014 / United States.