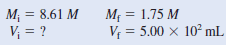Dilution of Solutions
– In this subject, we will discuss the Dilution of Solutions and Dilution Law.
Dilution of Solutions
– Concentrated solutions are often stored in the laboratory stockroom for use as needed.
– Frequently we dilute these “stock” solutions before working with them.
– Dilution of solution is the procedure for preparing a less concentrated solution from a more concentrated one.
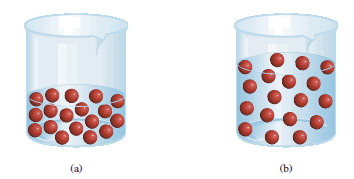
– Suppose that we want to prepare 1 L of a 0.400 M KMnO4 solution from a solution of 1.00 M KMnO4.
– For this purpose, we need 0.400 mole of KMnO4.
– Because there is 1.00 mole of KMnO4 in 1 L of a 1.00 M KMnO4 solution, there is 0.400 mole of KMnO4 in 0.400 L of the same solution:
– Therefore, we must withdraw 400 mL from the 1.00 M KMnO4 solution and dilute it to 1000 mL by adding water (in a 1-L volumetric flask).
– This method gives us 1 L of the desired solution of 0.400 M KMnO4
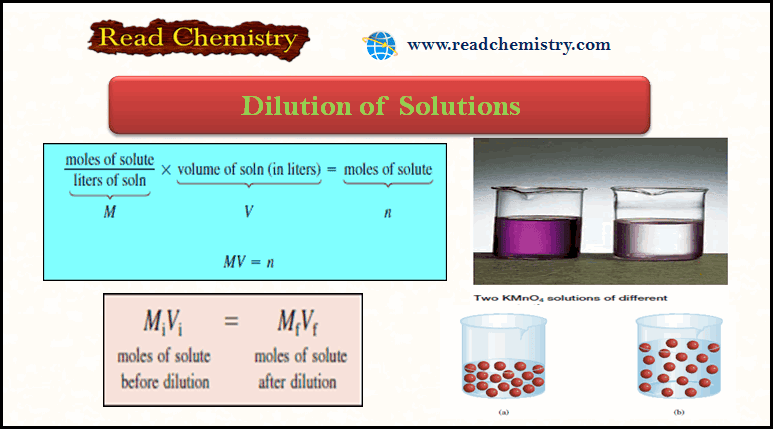
Dilution law
– In carrying out a dilution process, it is useful to remember that adding more solvent to a given amount of the stock solution changes (decreases) the concentration of the solution without changing the number of moles of solute present in the solution.
moles of solute before dilution = moles of solute after dilution
– Molarity is defined as moles of solute in one liter of solution, so the number of moles of solute is given by:
– Because all the solute comes from the original stock solution, we can conclude that n remains the same; that is,
– Mi and Mf are the initial and final concentrations of the solution in molarity, respectively
– Vi and Vf are the initial and final volumes of the solution, respectively.
– Of course, the units of Vi and Vf must be the same (mL or L) for the calculation to work.
– To check the reasonableness of your results, be sure that Mi > Mfand Vf > Vi.
Solved problems on Dilution of Solutions
Describe how you would prepare 5.00 × 102 mL of a 1.75 M H2SO4 solution, starting with an 8.61 M stock solution of H2SO4.
Strategy:
– Because the concentration of the final solution is less than that of the original one, this is a dilution process.
– Keep in mind that in dilution, the concentration of the solution decreases but the number of moles of the solute remains the same.
Solution:
– We prepare for the calculation by tabulating our data:
– Thus, we must dilute 102 mL of the 8.61 M H2SO4 solution with sufficient water to give a final volume of 5.00 × 102 mL in a 500-mL volumetric flask to obtain the desired concentration.
Check:
– The initial volume is less than the final volume, so the answer is reasonable.
- Reference: Chemistry / Raymond Chang, Williams College /(10th edition).