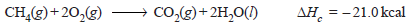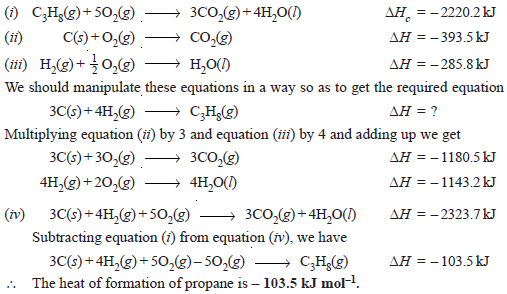Heat of Combustion (Definition, Applications, Solved Problems)
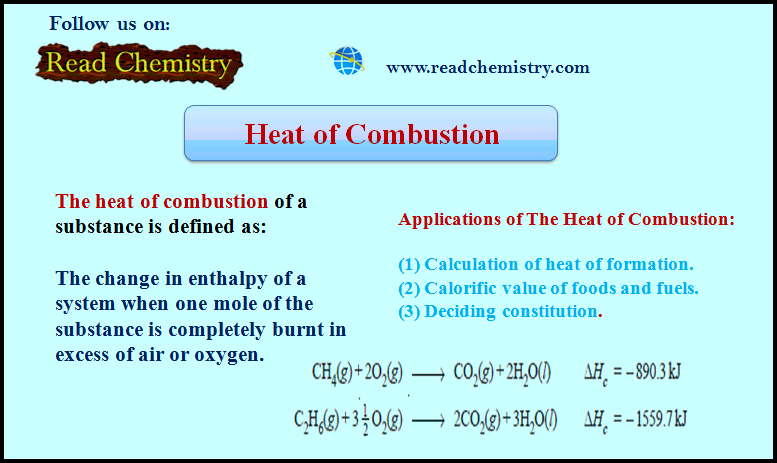
Heat of Combustion
– The heat of combustion of a substance is defined as The change in enthalpy of a system when one mole of the substance is completely burnt in excess of air or oxygen.
– It is denoted by ΔHc.
– For example, the heat of combustion of methane is – 21.0 kcal (= 87.78 kJ) as shown by the equation:
– Now consider the chemical equations:
– It may be noted that – 94.3 kcal and not – 26.0 kcal is the heat of combustion of carbon as the combustion is complete only in the first reaction.
– In the second case, oxidation has converted carbon to carbon monoxide and is by no means complete as carbon monoxide can be further oxidized to carbon dioxide.
– It should be noted clearly that the heat of combustion of a substance (ΔHc) is always negative.
– Heat energy is evolved during the process of combustion i.e., ΔHc = – ve.
Applications of The Heat of Combustion
(1) Calculation of heat of formation
– Since the heats of combustion of organic compounds can be determined with considerable ease, these are employed to calculate their heats of formation.
– The direct determination of these is often impossible
(2) Calorific value of foods and fuels
– The calorific value is defined as the amount of heat produced in calories (or joules) when one gram of a substance is completely burnt.
– It is expressed in cal g–1 or kcal g–1 or kJ g–1.
– Let us compare the calorific values of methane and ethane.
– Their heats of combustion are – 890.3 kJ and – 1559.7 kJ.
– These combustion reactions are expressed as:
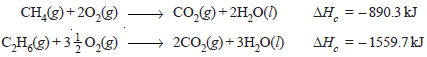
– In case of methane heat produced per gram is 890.3/16 = 55.64 kJ g–1 while for ethane it is –1559.7/30 = 51.90 kJ g–1.
– Thus methane has better fuel efficiency than ethane as it produces more heat per gram.
(3) Deciding constitution
– Heat of combustion of organic compounds is to a large extent an additive property, as shown by the fact that in a homologous series, the difference between the heats of combustion of successive members is nearly constant and is equal to 158 Cals.
– Constants corresponding to the heats of combustion of various atoms and linkages have been worked out.
– The heat of combustion of an organic substance can be calculated from its probable structural formula by adding up the values of the constants corresponding to the atoms and linkages involved therein.
– If the value so obtained comes out to be the same as the experimental value of the heat of combustion of the compound, the assumed formula must be correct.
– In this way Kekule’s formula for benzene with alternate double and single linkages has been supported as the calculated value of the heat of combustion of benzene according to this formula agrees with the actual heat of combustion.
– Heat of combustion of organic compounds has thus proved very valuable in deciding their chemical constitution.
Solved Problem
Calculate the standard heat of formation of propane (C3H8) if its heat of combustion is – 2220.2 kJ mol–1. The heats of formation of CO2(g) and H2O(l) are – 393.5 and – 285.8 kJ mol–1 respectively.
Solution
– We are given:
Reference: Essentials of Physical Chemistry /Arun Bahl, B.S Bahl and G.D. Tuli / multicolour edition.