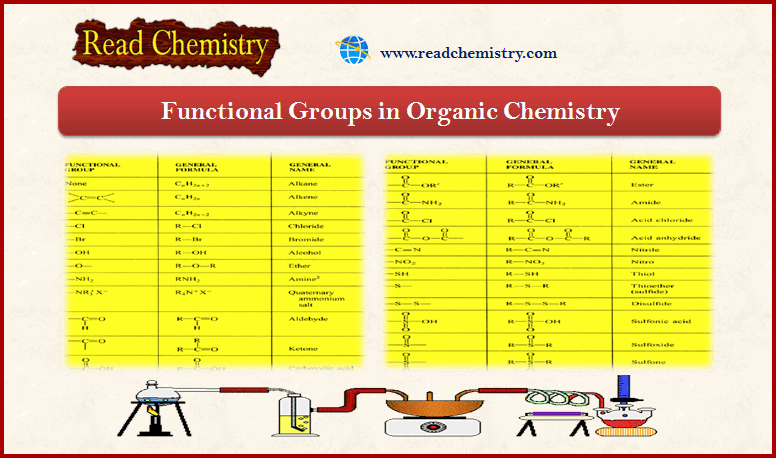
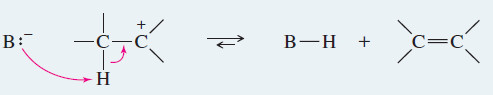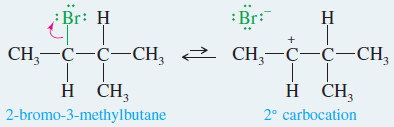E1 Reaction : First-Order Elimination
First-Order Elimination : The E1 Reaction
– An elimination involves the loss of two atoms or groups from the substrate, usually with formation of a pi bond.
– Elimination reactions frequently accompany and compete with substitutions.
– By varying the reagents and conditions, we can often modify a reaction to favor substitution or to favor elimination.
– First we will discuss eliminations by themselves.
– Then we consider substitutions and eliminations together, trying to predict what products and what mechanisms are likely with a given set of reactants and conditions.
– Depending on the reagents and conditions involved, an elimination might be a first-order (E1) or second-order (E2) process.
– The following examples illustrate the types of eliminations:
Mechanism and Kinetics of the E1 Reaction
– The abbreviation E1 stands for Elimination, unimolecular.
– The mechanism is called unimolecular because the rate-limiting transition state involves a single molecule rather than a collision between two molecules.
– The slow step of an E1 reaction is the same as in the SN1 reaction. unimolecular ionization to form a carbocation.
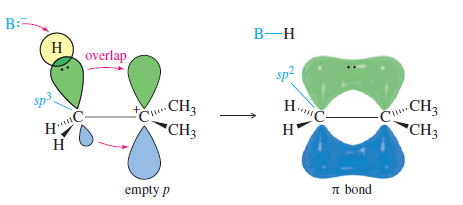
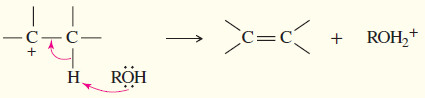
– In a fast second step, a base abstracts a proton from the carbon atom adjacent to the C+
– The electrons that once formed the carbon–hydrogen bond now form a pi bond between two carbon atoms.
– The general mechanism for the E1 reaction is shown in rhe following Key Mechanism:
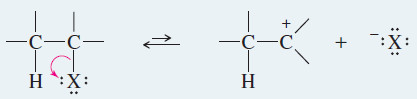
KEY MECHANISM: The E1 reaction
– The E1 reaction requires ionization to a carbocation intermediate like the SN1 , so it follows the same order of reactivity:
3° > 2° >> 1°.
– A base (usually weak) deprotonates the carbocation to give an alkene.
Step 1: Unimolecular ionization to give a carbocation (rate-limiting).
Step 2: Deprotonation by a weak base (often the solvent) gives the alkene (fast).
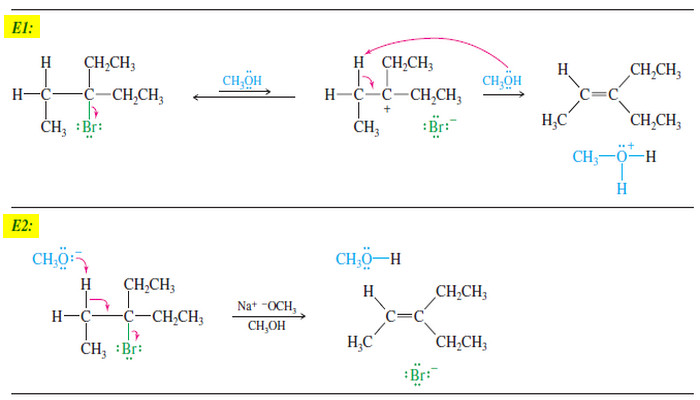
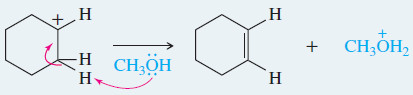
EXAMPLE: E1 elimination of bromocyclohexane in methanol.
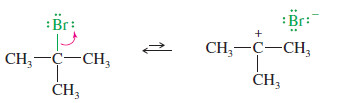
Step 1: Ionization gives a carbocation and bromide ion in a slow step.
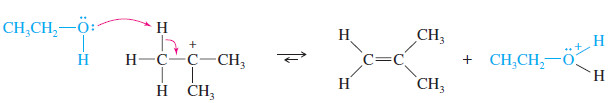
Step 2: Methanol abstracts a proton to give cyclohexene in a fast step.
– Because the rate-limiting step involves unimolecular ionization of the alkyl halide, the rate equation is first-order.
– The rate depends only on the concentration of the alkyl halide, and not on the strength or concentration of the base.
E1 rate = kr [RX]
– The weak base (often the solvent) takes part in the fast second step of the reaction.
Competition with the SN1 Reaction
– The E1 reaction almost always competes with the SN1 reaction.
– Whenever a carbocation is formed, it can undergo either substitution or elimination, and mixtures of products often result.
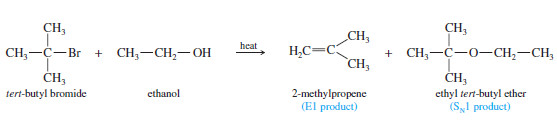
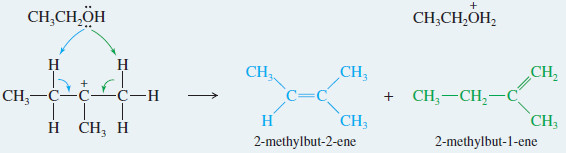
– The following reaction shows the formation of both elimination and substitution products in the reaction of tert-butyl bromide with boiling ethanol.
– The 2-methylpropene product results from dehydrohalogenation, an elimination of hydrogen and a halogen atom.
– Under these first-order conditions (the absence of a strong base), dehydrohalogenation takes place by the E1 mechanism: Ionization of the alkyl halide gives a carbocation intermediate, which loses a proton to give the alkene.
– Substitution results from nucleophilic attack on the carbocation.
– Ethanol serves as a base in the elimination and as a nucleophile in the substitution
Step 1: Ionization to form a carbocation.
Step 2: (by the E1 mechanism): Basic attack by the solvent abstracts a proton to give an alkene.
or, step 2 (by the SN1 mechanism): Nucleophilic attack by the solvent on the carbocation.
– Under ideal conditions, one of these first-order reactions provides a good yield of one product or the other.
– Often, however, carbocation intermediates react in two or more ways to give mixtures of products.
– For this reason, and E1 reactions of alkyl halides are not often used for organic synthesis.
– They have been studied in great detail to learn about the properties of carbocations, however.
Orbitals and Energetics of the E1 Reaction
– In the second step of the E1 mechanism, the carbon atom next to the C+ must rehybridize to sp2 as the base attacks the proton and electrons flow into the new pi bond
– The potential-energy diagram for the E1 reaction (see Figure) is similar to that for the SN1 reaction.
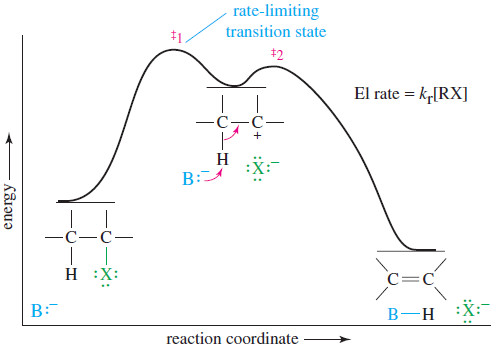
– The ionization step is strongly endothermic, with a rate-limiting transition state.
– The second step is a fast exothermic deprotonation by a base.
– The base is not involved in the reaction until after the rate-limiting step, so the rate depends only on the concentration of the alkyl halide.
– Weak bases are common in E1 reactions.
Rearrangements in E1 Reactions
– Like other carbocation reactions, the E1 may be accompanied by rearrangement.
– Compare the following E1 reaction (with rearrangement) with the SN1 reaction of the same substrate.
– Note that the solvent acts as a base in the E1 reaction and a nucleophile in the SN1 reaction.
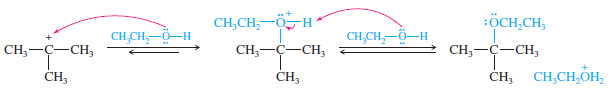
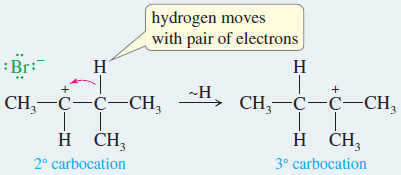
MECHANISM: Rearrangement in an E1 Reaction
– Like other reactions involving carbocations, the E1 may be accompanied by rearrangement.
Step 1: Ionization to form a carbocation. (slow)
Step 2: A hydride shift forms a more stable carbocation. (fast)
Step 3: The weakly basic solvent removes either adjacent proton. (fast)
SUMMARY Carbocation Reactions
A carbocation can:
1. React with its own leaving group to return to the reactant:
2. React with a nucleophile to form a substitution product (SN1):
3. Lose a proton to form an elimination product (an alkene) (E1):
4. Rearrange to a more stable carbocation, then react further.
– The order of stability of carbocations is: resonance-stabilized
3° > 2° > 1°.