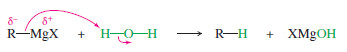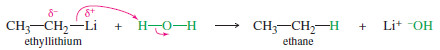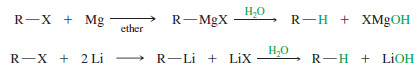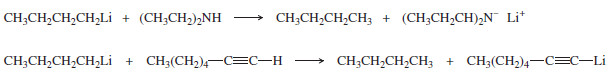Side Reactions of Organometallic Reagents
Side Reactions of Organometallic Reagents: Reduction of Alkyl Halides
– Organometallic Reagents: Grignard and organolithium reagents are strong nucleophiles and strong bases.
– Besides their additions to carbonyl compounds, they react with other acidic or electrophilic compounds.
– In some cases, these are useful reactions, but they are often seen as annoying side reactions where a small impurity of water or an alcohol destroys the reagent.
(A) Reactions with Acidic Compounds
– Grignard and organolithium reagents react vigorously and irreversibly with water.
– Therefore, all reagents and solvents used in these reactions must be dry.
For example, consider the reaction of ethyllithium with water:
– The products are strongly favored in this reaction. Ethane is a very weak acid ( Ka of about 10-50), so the reverse reaction (abstraction of a proton from ethane by lithium hydroxide) is unlikely.
– When ethyllithium is added to water, ethane instantly bubbles to the surface.
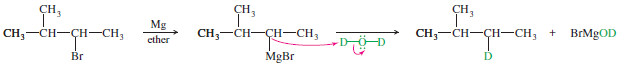
– Why would we ever want to add an organometallic reagent to water? This is a method for reducing an alkyl halide to an alkane:
– The overall reaction is a reduction because it replaces the electronegative halogen atom with a hydrogen atom.
– In particular, this reaction provides a way to “label” a compound with deuterium (D or 2H, heavy isotope of hydrogen) at any position where a halogen is present
– In addition to O-H groups, the protons of N-H and S-H groups and the hydrogen atom of a terminal alkyne, -C≡C-H are sufficiently acidic to protonate Grignard and organolithium reagents.
– Unless we want to protonate the reagent, compounds with these groups are considered incompatible with Grignard and organolithium reagents
(B) Reactions with Electrophilic Multiple Bonds
– Grignard reagents are useful because they add to the electrophilic double bonds of carbonyl groups. However, we must make sure that the only electrophilic double bond in the solution is the one we want the reagent to attack.
– There must be no electrophilic double (or triple) bonds in the solvent or in the Grignard reagent itself, or they will be attacked as well.
– Any multiple bond involving a strongly electronegative element is likely to be attacked, including and bonds.
– In later chapters, we will encounter methods for protecting susceptible groups to prevent the reagent from attacking them.
– For now, simply remember that the following groups react with Grignard and organolithium reagents; avoid compounds containing these groups except for the one carbonyl group that gives the desired reaction.
- Protonate the Grignard or organolithium: O-H, N-H, S-H, C≡C-H
- Attacked by the Grignard or organolithium: C=O, C=N, C≡N, S=O, N=O