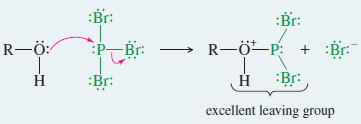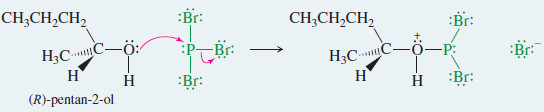Reaction of Alcohols with Phosphorus Halides
Reaction of Alcohols with Phosphorus Halides
– Reaction of Alcohols with Phosphorus Halides gives alkyl halides.
– Several phosphorus halides are useful for converting alcohols to alkyl halides.
– Phosphorus tribromide, phosphorus trichloride, and phosphorus pentachloride work well and are commercially available.
3 R-OH + PCl3 → 3 R-Cl + P(OH)3
3 R-OH + PBr3 → 3 R-Br + P(OH)3
R-OH + PCl5 → R-Cl + POCl3 + HCl
– Phosphorus triiodide is not sufficiently stable to be stored, but it can be generated in situ (in the reaction mixture) by the reaction of phosphorus with iodine.
2 P + 3 I2 ↔ 2 PI3
6 R-OH + 2 P + 3 I2 → 6 R-I + 2 P(OH)3
– Phosphorus halides produce good yields of most primary and secondary alkyl halides, but none works well with tertiary alcohols.
– The two phosphorus halides used most often are PBr3 and the phosphorus/iodine combination.
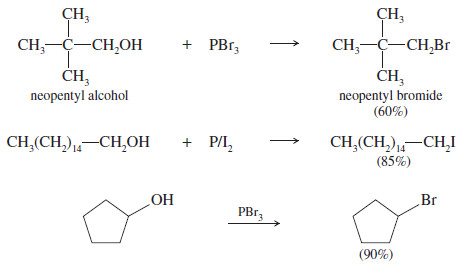
– Phosphorus tribromide is often the best reagent for converting a primary or secondary alcohol to the alkyl bromide, especially if the alcohol might rearrange in strong acid.
– A phosphorus and iodine combination is one of the best reagents for converting a primary or secondary alcohol to the alkyl iodide.
– For the synthesis of alkyl chlorides, thionyl chloride (discussed in the next section) generally gives better yields than PCl3 or PCl5 especially with tertiary alcohols.
– The following examples show the conversion of primary and secondary alcohols to bromides and iodides by treatment with PBr3 and P/I2.
Mechanism of the Reaction with Phosphorus Trihalides
– The mechanism of the reaction of alcohols with phosphorus trihalides explains why rearrangements are uncommon and why phosphorus halides work poorly with tertiary alcohols.
– The mechanism is shown here using PBr3 as the reagent; PCl3 and PI3 (generated from phosphorus and iodine) react in a similar manner
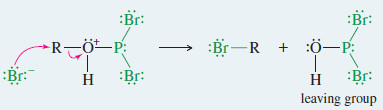
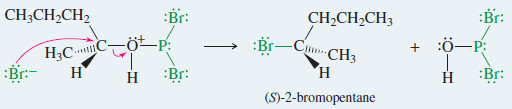
Mechanism: Reaction of Alcohols with PBr3
Step 1: PBr3 is a strong electrophile. An alcohol displaces bromide ion from PBr3 to give an excellent leaving group.
Step 2: Bromide displaces the leaving group to give the alkyl bromide.
Example: Reaction of (R)-pentan-2-ol with PBr3.
Step 1: Displacement of bromide and formation of a leaving group.
Step 2: Bromide displaces the leaving group to give (S)-2-bromopentane.
– Rearrangements are uncommon because no carbocation is involved, so there is no opportunity for rearrangement.
– This mechanism also explains the poor yields with tertiary alcohols.
– The final step SN2 is an displacement where bromide attacks the back side of the alkyl group.
– This attack is hindered if the alkyl group is tertiary.
– In the case of a tertiary alcohol, an ionization to a carbocation is needed.
– This ionization is slow, and it invites side reactions.