Characteristic Absorptions of Carbonyl Compounds
– In this subject, we will talk about Characteristic Absorptions of Carbonyl Compounds such as Ketones, Aldehydes, Amines, and Acids.
Characteristic Absorptions of Carbonyl Compounds
– Because it has a large dipole moment, the C=O double bond produces intense infrared stretching absorptions.
– Carbonyl groups absorb at frequencies around 1700 cm-1 but the exact frequency depends on the specific functional group and the rest of the molecule.
– For these reasons, infrared spectroscopy is often the best method for detecting and identifying the type of carbonyl group in an unknown compound.
– To simplify our discussion of carbonyl absorptions, we first consider the (normal) stretching frequencies for simple ketones, aldehydes, and carboxylic acids, and then we examine the types of carbonyl groups that deviate from this frequency.
(1) Simple Ketones, Aldehydes, and Acids
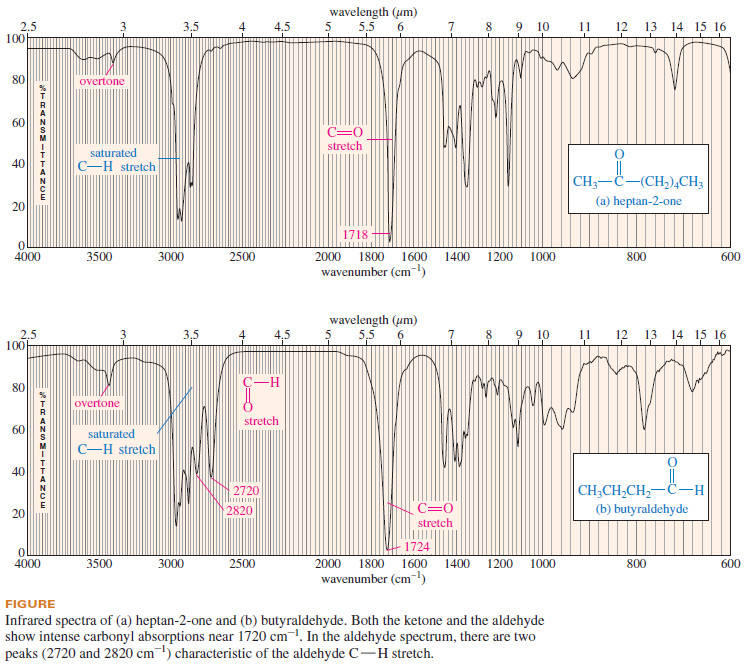
– The C=O stretching vibrations of simple ketones and carboxylic acids occur at frequencies around 1710 cm-1.
– Aldehydes are a little higher, about 1725 cm-1.
– These frequencies are higher than those for C=C double bonds because the C=O double bond is stronger and stiffer.
– Carbonyl absorptions may be so intense that they produce small overtone peaks around 3400 cm-1, double their fundamental frequency.
– In addition to the strong C=O stretching absorption, an aldehyde shows a characteristic set of two low-frequency C-H stretching frequencies around 2700 and 2800 cm-1 Neither a ketone nor an acid produces absorptions at these positions.
– The following Figure compares the IR spectra of a ketone and an aldehyde.
– Notice the characteristic carbonyl stretching absorptions in both spectra, as well as the aldehyde C-H absorptions at 2720 and 2820 cm-1 in the butyraldehyde spectrum.
– Both spectra in the previous Figure also show small overtone peaks around 3400 cm-1, double their carbonyl frequencies.
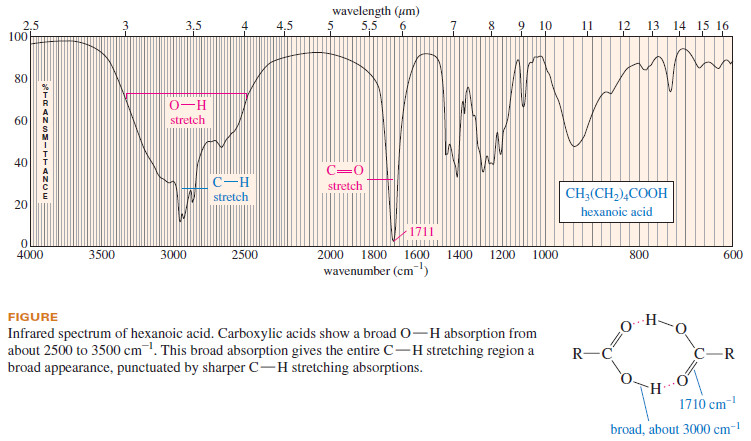
– A carboxylic acid produces a characteristic broad O-H absorption in addition to
the intense carbonyl stretching absorption (see the following Figure).
– Because of the unusually strong hydrogen bonding in carboxylic acids, the broad O-H stretching frequency is shifted to about 3000 cm-1, centered on top of the usual C-H absorption.
– This broad O-H absorption (which may have a shoulder or small spikes around 2500–2700 cm-1) gives a characteristic overinflated shape to the peaks in the C-H stretching region.
– Participation of the acid carbonyl group in hydrogen bonding frequently results in broadening of the strong carbonyl absorption as well.
(2) Resonance Lowering of Carbonyl Frequencies
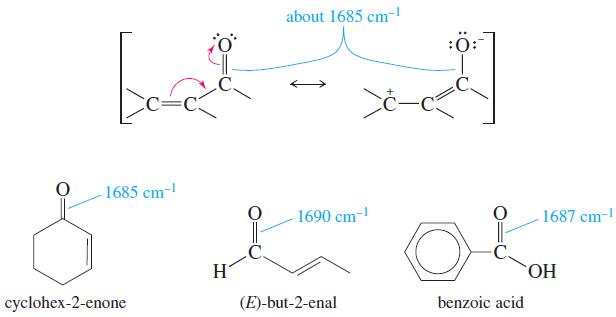
– we saw before that the conjugation of a C=C double bond lowers its stretching frequency.
– This is also true of conjugated carbonyl groups, as shown next.
– Delocalization of the pi electrons reduces the electron density of the carbonyl double bond, weakening it and lowering the stretching frequency from about 1710 cm-1 to about 1685 cm-1 for conjugated ketones, aldehydes, and acids.
– The C=C absorption of a conjugated carbonyl compound may not be apparent in the IR spectrum because it is so much weaker than the C=O absorption.
– The presence of the C=C double bond can still be inferred from its effect on the C=O frequency and the presence of unsaturated =C-H absorptions above 3000 cm-1
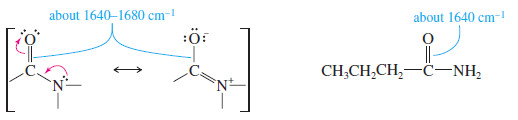
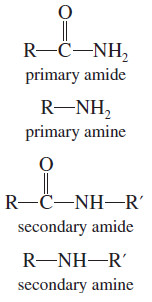
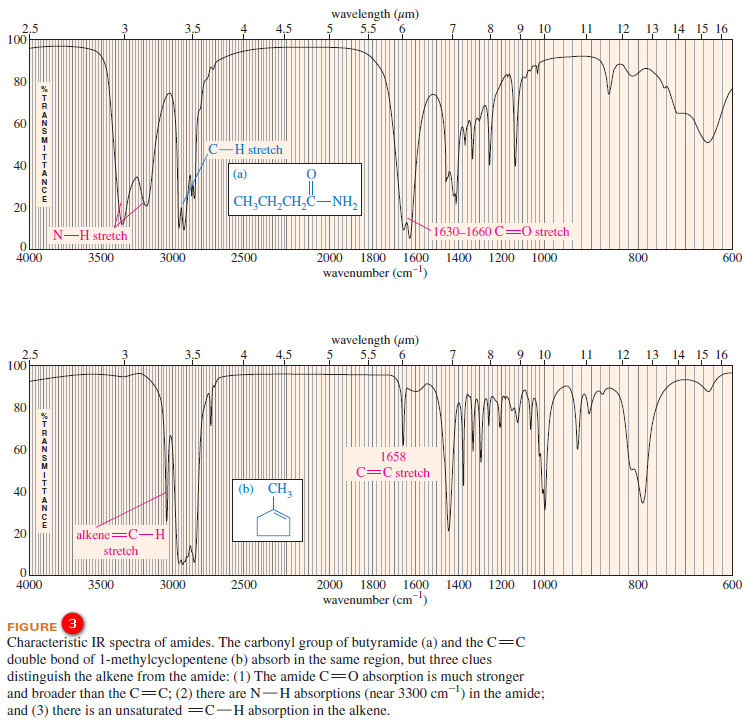
– The carbonyl groups of amides absorb at particularly low IR frequencies: about 1640 to 1680 cm-1 (see Figure 3).
– The dipolar resonance structure (shown next) places part of the pi bond between carbon and nitrogen, leaving less than a full C=O double bond.
– The very low frequency of the amide carbonyl might be mistaken for an alkene C=C stretch.
– For example, consider the spectra of butyramide ( C=O about 1640 cm-1) and 1-methylcyclopentene (C=C at 1658 cm-1 ) in Figure 12-13. Three striking differences are evident in these spectra:
(1) The amide carbonyl absorption is much stronger and broader (from hydrogen bonding) than the absorption of the alkene double bond;
(2) There are prominent N-H stretching absorptions in the amide spectrum; and
(3) There is an unsaturated C-H stretching (just to the left of 3000 cm-1) in the alkene spectrum.
– These examples show that we can distinguish between C=O and C=C absorptions, even when they appear in the same part of the spectrum.
Absorptions of Carbonyl Compounds in Amines
– Like primary amines, most primary amides show two spikes in the N-H stretching region (about 3300 cm-1), as in the butyramide spectrum (Figure 3).
– Secondary amides (like secondary amines) generally show one N-H spike.
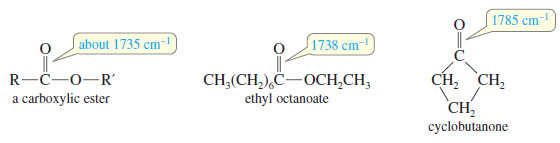
(3) Carbonyl Absorptions Above 1725 cm-1
– Some carbonyl groups absorb at frequencies higher than 1725 cm-1
– For example, simple carboxylic esters absorb around 1725 cm-1
These higher-frequency absorptions are also seen in strained cyclic ketones (in a five-membered ring or smaller).
– In a small ring, the angle strain on the carbonyl group forces more electron density into the C=O double bond, resulting in a stronger, stiffer bond.