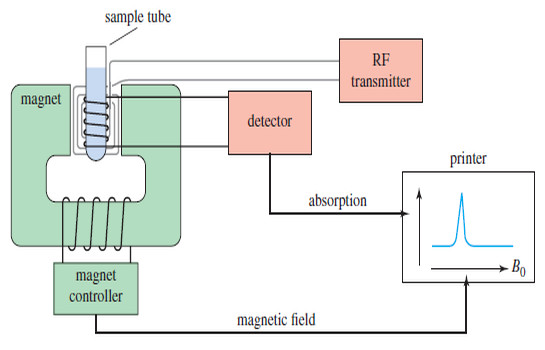
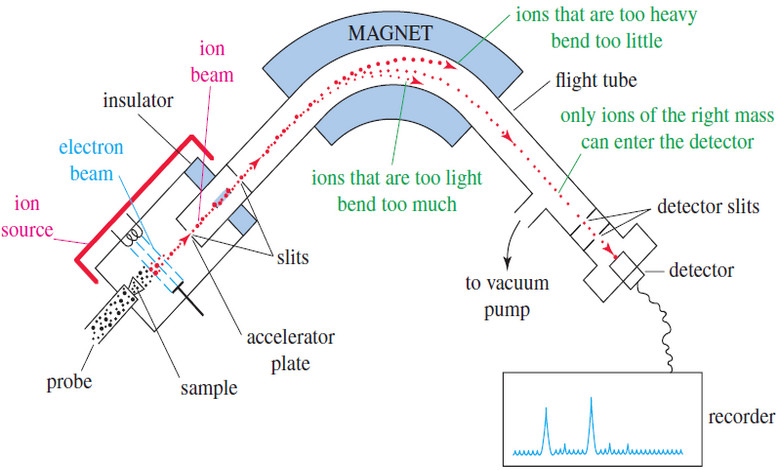
NMR spectrometer
What happens in an NMR spectrometer?
– Before discussing the design of spectrometers, let’s review what happens in an NMR spectrometer.
– Protons (in the sample compound) are placed in a magnetic field, where they align either with the field or against it.
– While still in the magnetic field, the protons are subjected to radiation of a frequency they can absorb by changing the orientation of their magnetic moment relative to the field.
– If protons were isolated, they would all absorb at the same frequency, proportional to the magnetic field.
– But protons in a molecule are partially shielded from the magnetic field, and this shielding depends on each proton’s environment.
– Thus, protons in different environments within a molecule exposed to a constant frequency absorb the radiation at different magnetic field strengths.
– The NMR spectrometer was originally developed to vary the magnetic field and plot a graph of energy absorption as a function of the magnetic field strength.
– Such a graph is called a nuclear magnetic resonance spectrum.
NMR spectrometer
– The original, simplest type of NMR spectrometer consisted of four parts:
(1) A stable magnet, with a sensitive controller to produce a precise magnetic field.
(2) A radio-frequency (RF) transmitter, emitting a precise frequency (continous wave or CW).
(3) A detector to measure the sample’s absorption of RF energy.
(4) A recorder to plot the output from the detector versus the applied magnetic field.
– The printer records a graph of absorption (on the y axis) as a function of the applied magnetic field (on the x axis).
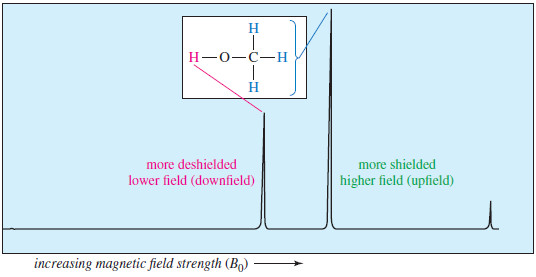
– Higher values of the magnetic field are toward the right (upfield), and lower values are toward the left (downfield).
– The absorptions of more shielded protons appear upfield, toward the right of the spectrum, and more deshielded protons appear downfield, toward the left.
– The NMR spectrum of methanol is shown in the following Figure:
– The previous figure shows Proton NMR spectrum of methanol.
– The more shielded methyl protons appear toward the right of the spectrum (higher field); the less shielded hydroxyl proton appears toward the left (lower field).