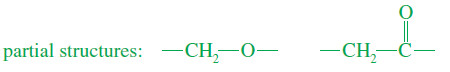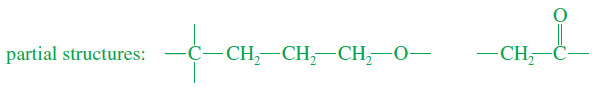Interpreting Carbon NMR Spectra
Interpreting Carbon NMR Spectra
– Interpreting Carbon NMR Spectra (13C NMR spectra) uses the same principles as interpreting 1H NMR spectra.
– In fact, carbon spectra are often easier to interpret.
– The 13C NMR spectrum provides the following information:
(1) The number of different signals implies how many different types of carbons are present.
(2) The chemical shifts of those signals suggest what types of functional groups contain those carbon atoms.
(3) The splitting of signals in the off-resonance-decoupled spectrum or the DEPT-90 and DEPT-135 spectra indicate how many protons are bonded to each carbon atom.
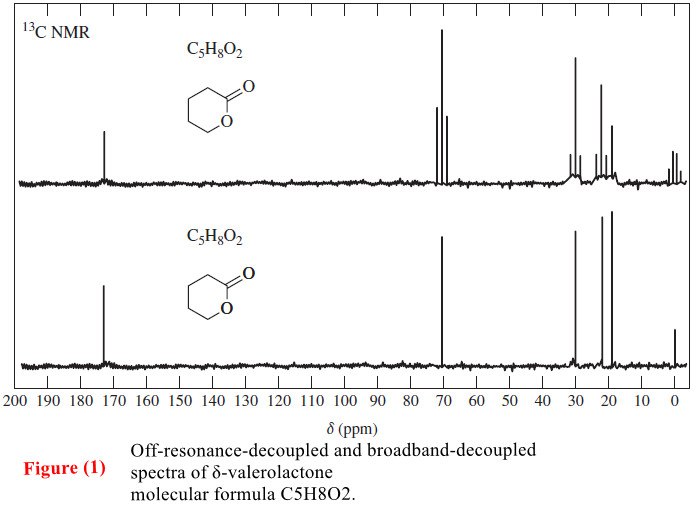
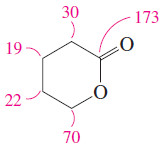
– For example, in the 13C NMR spectrum of δ-valerolactone (Figure 1), the groups in the upper (off-resonance decoupled) spectrum are split into triplets, but they appear as singlets in the lower (broadband-decoupled) spectrum.
– Let’s consider how we might solve this structure, given only the 13C NMR spectrum and the molecular formula.
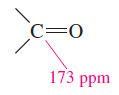
– As we have seen in Figures (2) and (3) in the last subject we discuss (Carbon-13 NMR Spectroscopy), the signal at 173 ppm is appropriate for a carbonyl carbon.
– The off-resonance-decoupled spectrum shows a singlet at 173 ppm, implying that no hydrogens are bonded to the carbonyl carbon.
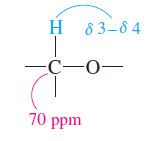
– The chemical shift of the next absorption is about 70 ppm.
– This is about 20 times the chemical shift of a proton on a carbon bonded to an electronegative element.
– The molecular formula implies that the electronegative element must be oxygen.
– Since the signal at 70 ppm is a triplet in the off-resonance-decoupled spectrum, this carbon must be a methylene (-CH2-) group.
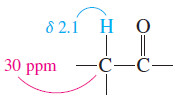
– The signal at 30 ppm corresponds to a carbon atom bonded to a carbonyl group.
– Remember that a proton on a carbon adjacent to a carbonyl group absorbs around 2.1 ppm, and we expect the carbon to have a chemical shift about 15 to 20 times as large.
– This carbon atom is a methylene group, as shown by the triplet in the offresonance– decoupled spectrum.
– The two signals at 19 and 22 ppm are from carbon atoms that are not directly bonded to any deshielding group, although the carbon at 22 ppm is probably closer to one of the oxygen atoms.
– These are also triplets in the off-resonance-decoupled spectrum and they correspond to methylene groups.
– We can propose:
– The molecular formula C5H8O2 implies the presence of two elements of unsaturation.
– The carbonyl (C=O) group accounts for one, but there are no more carbonyl groups and no double-bonded alkene carbon atoms.
– The other element of unsaturation must be a ring.
– Combining the partial structures into a ring gives the complete structure.
References:
- Organic chemistry / L.G. Wade, Jr / 8th ed, 2013 / Pearson Education, Inc. USA.
- Fundamental of Organic Chemistry / John McMurry, Cornell University/ 8th ed, 2016 / Cengage Learningm, Inc. USA.
- Organic Chemistry / T.W. Graham Solomons, Craig B. Fryhle , Scott A. Snyder / 11 ed, 2014/ John Wiley & Sons, Inc. USA.