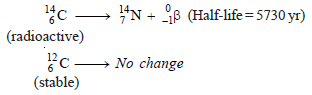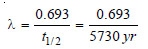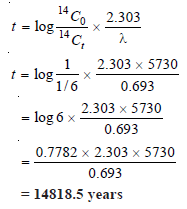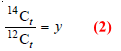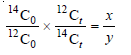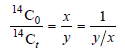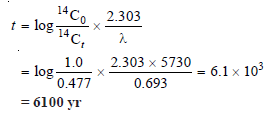Radioactive Dating: Definition, Formula, Examples
– In this subject, we will discuss the Radioactive Dating: Definition, Formula, Examples
Radioactivity
– Several elements such as uranium and radium are unstable.
– Their atomic nucleus breaks off its own accord to form a smaller atomic nucleus of another element.
– The protons and neutrons in the unstable nucleus regroup to give the new nucleus.
– This causes the release of excess particles and energy from the original nucleus, which we call radiation.
– The elements whose atomic nucleus emits radiation are said to be radioactive.
Radioactive Dating
– The age of an old piece of wood can be determined by radioactive dating techniques.
– The atmosphere contains radioactive carbon dioxide, 14CO2, and ordinary carbon dioxide, 12CO2, in a fixed ratio.
– A plant while alive takes up both types of carbon dioxide and converts them to carbon-14 and carbon-12 photosynthesis.
– Thus a living plant contains radioactive carbon-14 and stable carbon-12 in a fixed ratio.
– When the plant dies, the uptake of carbon from the atmosphere stops.
– From now onward, carbon-12 remains unchanged but carbon-14 decays by beta emission.
– As a result, 14C/ 12C decreases with lapse of time.
– Therefore the concentration of carbon-14 declines with time.
– The concentration of carbon-14 can be measured by counting radioactivity.
– Knowing the concentration of carbon-14 in a given sample of old wood and that in a living plant, the age of the sample can be calculated.
Solved Problems on Radioactive Dating
Problem(1)
The amount of carbon-14 in a piece of wood is found to be one-sixth of its amount in a fresh piece of wood. Calculate the age of an old piece of wood.
Solution:
– Calculation of Decay constant :
The half-life of carbon-14 = 5730 years
– Calculation of Age of Wood :
– In a new method for determining the age of old wood (or fossil), the measurement of radioactivity is avoided.
– The ratio 14C/12C is found with the help of a mass spectrometer in the old wood and fresh wood from a living plant.
– It is assumed that the ratio of 14C/12C in the fresh wood today is the same as it was at the time of the death of the plant.
– Let the ratio in the fresh plant at t = 0 be
– Let the ratio in the old piece of wood at time t be
– Dividing (1) by (2):
– The concentration of carbon-12 does not change with time and 12C0 = 12Ct
– Therefore
– where the ratio in the old wood is y/x times the ratio in the living plant.
– Knowing the value of y/x, the value of t can be found from the expression:
Problem (2)
A bone taken from a garbage pile buried under a hillside had a 14C/12C ratio 0.477 times the ratio in a living plant or animal. What was the date when the animal was buried?
Solution:
– The half-life of carbon-14 is 5730 years
– Substituting values in the expression
– The animal was buried 6100 years ago.
Reference: Essentials of Physical Chemistry /Arun Bahl, B.S Bahl and G.D. Tuli / multicolor edition.