Thermodynamics – Basic terms and concepts in Thermodynamics
What is Thermodynamics?
– Thermodynamics is The study of the flow of heat or any other form of energy into or out of a system as it undergoes a physical or chemical transformation.
– In studying and evaluating the flow of energy into or out of a system, it will be useful to consider changes in certain properties of the system.
– These properties include temperature, pressure, volume and concentration of the system.
– Measuring the changes in these properties from the initial state to the final state can provide information concerning changes in energy and related quantities such as heat and work.
– The study of thermodynamics is based on three broad generalisations derived from well-established experimental results.
– These generalisations are known as the First, Second and Third law of thermodynamics.
– These laws have stood the test of time and are independent of any theory of the atomic or molecular structure.
Scope of Thermodynamics
(1) Most of the important laws of Physical Chemistry, including the van’t Hoff law of lowering of vapour pressure, the Phase Rule and the Distribution Law, can be derived from the laws of thermodynamics.
(2) It tells whether a particular physical or chemical change can occur under a given set of conditions of temperature, pressure and concentration.
(3) It also helps in predicting how far a physical or chemical change can proceed until the equilibrium conditions are established.
Limitations of Thermodynamics
(1) Thermodynamics is applicable to macroscopic systems consisting of matter in bulk and not to microscopic systems of individual atoms or molecules.
– It ignores the internal structure of atoms and molecules.
(2) Thermodynamics does not bother about the time factor. That is, it does not tell anything regarding the rate of a physical change or a chemical reaction. It is concerned only with the initial and the final states of the system.
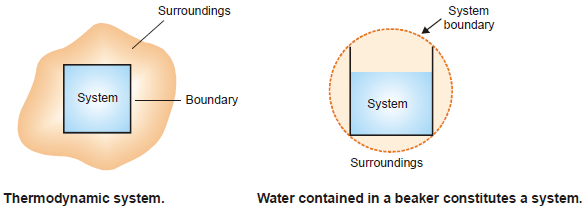
System, Boundary, Surroundings
– A system is that part of the universe which is under thermodynamic study and the rest of the universe is surroundings.
– The boundary is The real or imaginary surface separating the system from the surroundings.
– In experimental work, a specific amount of one or more substances constitutes the system.
– Thus 200 g of water contained in a beaker constitutes a thermodynamic system.
– The beaker and the air in contact, are the surroundings.
– Similarly, 1 mole of oxygen confined in a cylinder fitted with a piston, is a thermodynamic system.
– The cylinder and the piston and all other objects outside the cylinder form the surroundings.
– Here the boundary between the system (oxygen) and the surroundings (cylinder and piston) is clearly defined.
Homogeneous And Heterogeneous Systems
– When a system is uniform throughout, it is called a Homogeneous System.
– Examples of homogeneous systems are a pure single solid, liquid or gas, mixtures of gases, and true solution of a solid in a liquid.
– A homogeneous system is made of one phase only.
– A phase is defined as a homogeneous, physically distinct and mechanically separable portion of a system.
– A heterogeneous system is one which consists of two or more phases. In other words, it is not uniform throughout.
– Examples of heterogeneous systems are ice in contact with water, ice in contact with vapour etc. Here ice, water and vapour constitute separate phases.
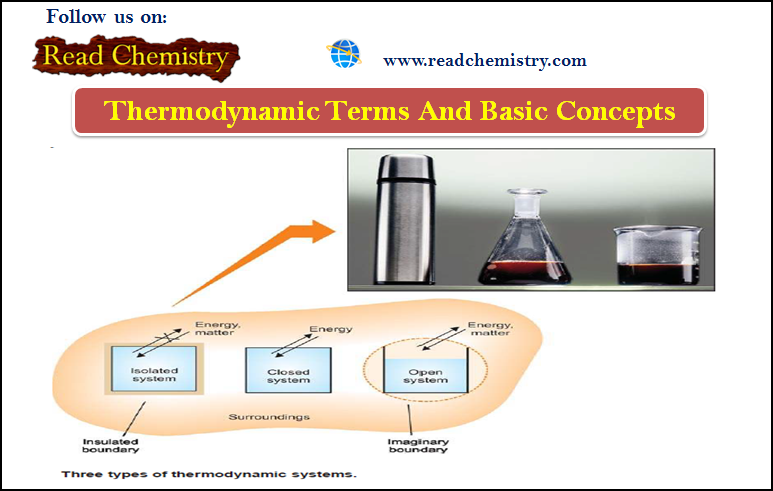
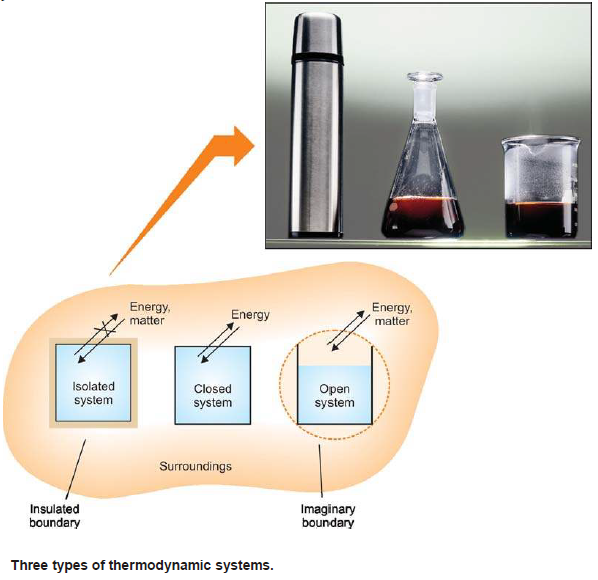
Types Of Thermodynamic Systems
– There are three types of thermodynamic systems depending on the nature of the boundary:
(1) Isolated System
(2) Closed System
(3) Open System
– If the boundary is closed or sealed, no matter can pass through it.
– If the boundary is insulated, no energy (say heat) can pass through it.
(1) Isolated System
– When the boundary is both sealed and insulated, no interaction is possible with the surroundings.
– Therefore, an isolated system is one that can transfer neither matter nor energy to and from its surroundings.
– Let us consider a system of 100 ml of water in contact with its vapour in a closed vessel which is insulated.
– Since the vessel is sealed, no water vapour (matter) can escape from it.
– Also, because the vessel is insulated, no heat (energy) can be exchanged with the surroundings.
– A substance, say boiling water, contained in a thermos flask, is another example of an isolated system.
(2) Closed System
– Here the boundary is sealed but not insulated.
– Therefore, a closed system is one which cannot transfer matter but can transfer energy in the form of heat, work and radiation to and from its surroundings.
– A specific quantity of hot water contained in a sealed tube is an example of a closed system.
– While no water vapour can escape from this system, it can transfer heat through the walls of the tube to the surroundings.
– A gas contained in a cylinder fitted with a piston constitutes a closed system.
– As the piston is raised, the gas expands and transfers heat (energy) in the form of work to the surroundings.
(3) Open System
– In such a system the boundary is open and un-insulated.
– Therefore, an open system is one which can transfer both energy and matter to and from its surroundings.
– Hot water contained in a beaker placed on a laboratory table is an open system.
– The water vapour (matter) and also heat (energy) is transferred to the surroundings through the imaginary boundary.
– Zinc granules reacting with dilute hydrochloric acid to produce hydrogen gas in a beaker is another example of an open system.
– Hydrogen gas escapes and the heat of the reaction is transferred to the surroundings.
What are adiabatic Systems?
– Those systems in which no thermal energy passes into or out of the system, are said to be adiabatic systems.
Intensive and Extensive Properties
– The macroscopic or bulk properties of a system (volume, pressure, mass, etc.) can be divided into two classes:
(a) Intensive properties
(b) Extensive properties
Intensive Properties
– Intensive Property is a property which does not depend on the quantity of matter present in the system.
– Some examples of intensive properties are pressure, temperature, density, and concentration.
– If the overall temperature of a glass of water (our system) is 20ºC, then any drop of water in that glass has a temperature of 20ºC.
– Similarly, if the concentration of salt, NaCl, in the glass of water, is 0.1 mole/litre, then any drop of water from the glass also has a salt concentration of 0.1 mole/litre.
Extensive Properties
– Extensive Property is A property that does depend on the quantity of matter present in the system.
– Some examples of extensive properties are volume, number of moles, enthalpy, entropy, and Gibbs’ free energy.
– Some of these properties are unfamiliar to you but these will be defined and illustrated later.
– By definition, the extensive properties are additive while intensive properties are not.
– Let us consider the system ‘a glass of water’.
– If we double the mass of water, the volume is doubled and so is the number of moles and the internal energy of the system.
State of A System
– A thermodynamic system is said to be in a certain state when all its properties are fixed.
– The fundamental properties which determine the state of a system are pressure (P), temperature (T), volume (V), mass and composition.
– Since a change in the magnitude of such properties alters the state of the system, these are referred to as State variables or State functions or Thermodynamic parameters .
– It also stands to reason that a change of system from the initial state to the final state (2nd state) will be accompanied by a change in the state variables.
– It is not necessary to state all the properties (state variables) to define a system completely.
– For a pure gas, the composition is fixed automatically, as it is cent per cent.
Equation of State
– The remaining state variables P, V, and T are interrelated in the form of an algebraic relationship called the Equation of State.
– Thus for one mole of a pure gas, the equation of state is:
PV = RT
where R is gas constant.
– If of the three state variables (P, V, T), P and T are specified, the value of the third (V) is fixed automatically and can be calculated from the equation of state.
– The variables (P and T) which must be necessarily specified to define the state of a system, are designated as Independent state variables.
– The remaining state variable (V) which depends on the value of P and T, is called the Dependent state variable.
– An important characteristic of a state variable (or state function) is that when the state of a system is altered, the change in the variable depends on the initial and final states of the system.
– For example, if we heat a sample of water from 0º C to 25º C, the change in temperature is equal to the difference between the initial and final temperatures.
ΔT = Tfinal – Tinitial = 25 ºC
– The way in which the temperature change is brought about has no effect on the result.
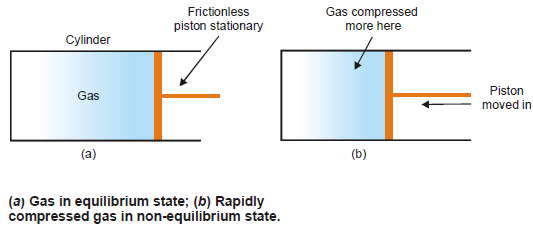
Equilibrium and Non-equilibrium states
– A system in which the state variables have constant values throughout the system is said to be in a state of thermodynamic equilibrium.
– Suppose we have a gas confined in a cylinder that has a frictionless piston.
– If the piston is stationary, the state of the gas can be specified by giving the values of pressure and volume.
– The system is then in a state of equilibrium.
– A system in which the state variables have different values in different parts of the system is said to be in a non-equilibrium state.
– If the gas contained in a cylinder, as stated above, is compressed very rapidly by moving down the piston, it passes through states in which pressure and temperature cannot be specified, since these properties vary throughout the gas.
– The gas near the piston is compressed and heated and that at the far end of the cylinder is not.
– The gas then would be said to be in a non-equilibrium state.
– Thermodynamics is concerned only with equilibrium states.
The Criteria for Equilibrium
(1) The temperature of the system must be uniform and must be the same as the temperature of the surroundings (thermal equilibrium).
(2) The mechanical properties must be uniform throughout the system (mechanical equilibrium). That is, no mechanical work is done by one part of the system on any other part of the system.
(3) The chemical composition of the system must be uniform with no net chemical change (chemical equilibrium).
– If the system is heterogeneous, the state variables of each phase remain constant in each phase.
Reference: Essentials of Physical Chemistry /Arun Bahl, B.S Bahl and G.D. Tuli / multicolour edition.