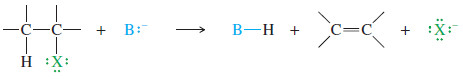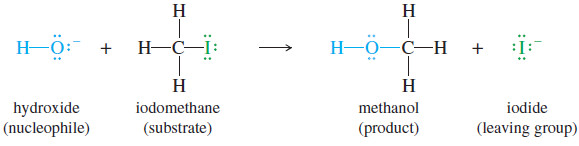SN2 reaction of Alkyl halides
In this subject Second-Order Nucleophilic Substitution: The SN2 Reaction of Alkyl halides will be discussed
Reactions of Alkyl Halides: Substitution and Elimination
– Alkyl halides are easily converted to many other functional groups.
– The halogen atom can leave with its bonding pair of electrons to form a stable halide ion; we say that a halide is a good leaving group.
– When another atom replaces the halide ion, the reaction is a substitution.
– When the halide ion leaves with another atom or ion (often H+) and forms a new pi bond, the reaction is an elimination.
– In many eliminations, a molecule of H-X is lost from the alkyl halide to give an alkene.
– These eliminations are called dehydrohalogenations because a hydrogen halide has been removed from the alkyl halide.
– Substitution and elimination reactions often compete with each other.
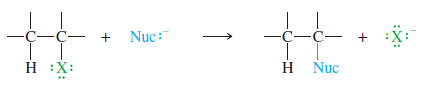
– In a nucleophilic substitution, a nucleophile (Nuc:–) replaces a leaving group (X–) from a carbon atom, using its lone pair of electrons to form a new bond to the carbon atom
Nucleophilic substitution
In an elimination, both the halide ion and another substituent are lost. A new bond is formed
Elimination
– In the elimination (a dehydrohalogenation), the reagent reacts as a base, abstracting a proton from the alkyl halide.
– Most nucleophiles are also basic and can engage in either substitution or elimination, depending on the alkyl halide and the reaction conditions.
– Besides alkyl halides, many other types of compounds undergo substitution and elimination reactions.
– Substitutions and eliminations are introduced in this subject using the alkyl halides as examples.
– In later subjects, we encounter substitutions and eliminations of other types of compounds.
Second-Order Nucleophilic Substitution: The SN2 Reaction
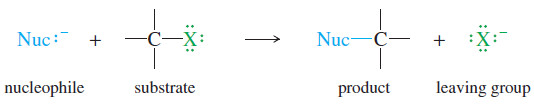
– A nucleophilic substitution has the general form.
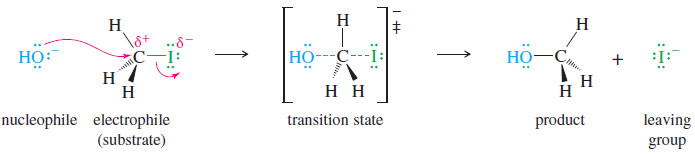
where Nuc:– is the nucleophile and X− is the leaving halide ion. An example is the reaction of iodomethane (CH3I) with hydroxide ion. The product is methanol.
– Hydroxide ion is a strong nucleophile (donor of an electron pair) because the oxygen atom has unshared pairs of electrons and a negative charge.
– Iodomethane is called the substrate, meaning the compound that is attacked by the reagent.
– The carbon atom of iodomethane is electrophilic because it is bonded to an electronegative iodine atom.
– Electron density is drawn away from carbon by the halogen atom, giving the carbon atom a partial positive charge.
– The negative charge of hydroxide ion is attracted to this partial positive charge
– Hydroxide ion attacks the back side of the electrophilic carbon atom, donating a pair of electrons to form a new bond. (In general, nucleophiles are said to attack electrophiles, not the other way around.)
– Notice that curved arrows are used to show the movement of electron pairs, from the electron-rich nucleophile to the electron poor carbon atom of the electrophile.
– Carbon can accommodate only eight electrons in its valence shell, so the carbon–iodine bond must begin to break as the carbon–oxygen bond begins to form.
– Iodide ion is the leaving group; it leaves with the pair of electrons that once bonded it to the carbon atom.
– This one-step mechanism is supported by kinetic information. One can vary the concentrations of the reactants and observe the effects on the reaction rate (how much methanol is formed per second).
– The rate is found to double when the concentration of either reactant is doubled.
– The reaction is therefore first order in each of the reactants and second order overall.
– The rate equation has the following form:
– This rate equation is consistent with a mechanism that requires a collision between a molecule of methyl iodide and a hydroxide ion.
– Both of these species are present in the transition state, and the collision frequency is proportional to both concentrations.
– The rate constant Kr depends on several factors, including the energy of the transition state and the temperature
– This one-step nucleophilic substitution is an example of the SN2 mechanism.
– The abbreviation SN2 stands for Substitution, Nucleophilic, bimolecular.
– The term bimolecular means that the transition state of the rate-limiting step (the only step in this reaction) involves the collision of two molecules.
– Bimolecular reactions usually have rate equations that are second order overall.
– The SN2 reaction of methyl iodide (iodomethane) with hydroxide ion is a concerted reaction, taking place in a single step with bonds breaking and forming at the same time.
– The middle structure is a transition state, a point of maximum energy, rather than an intermediate.
– In this transition state, the bond to the nucleophile (hydroxide) is partially formed, and the bond to the leaving group (iodide) is partially broken.
– Remember that a transition state is not a discrete molecule that can be isolated; it exists for only an instant.
– The reaction-energy diagram for this substitution (see Figure) shows only one transition state and no intermediates between the reactants and the products.
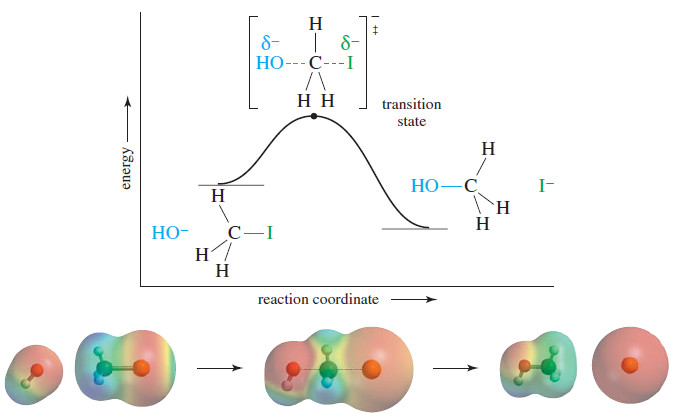
– The reactants are shown slightly higherin energy than the products because this reaction is known to be exothermic.
– The transition state is much higher in energy because it involves a fivecoordinate carbon atom with two partial bonds.
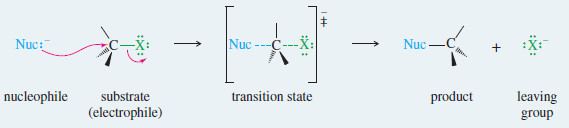
– The following mechanism shows a general SN2 reaction.
– A nucleophile attacks the substrate to give a transition state in which a bond to the nucleophile is forming at the same time as the bond to the leaving group is breaking.
MECHANISM: The SN2 Reaction
– The SN2 reaction takes place in a single (concerted) step.
– A strong nucleophile attacks the electrophilic carbon, forcing the leaving group to leave
The order of reactivity for substrates is:
CH3 X > 1° > 2°
(3° alkyl halides cannot react by this mechanism.)
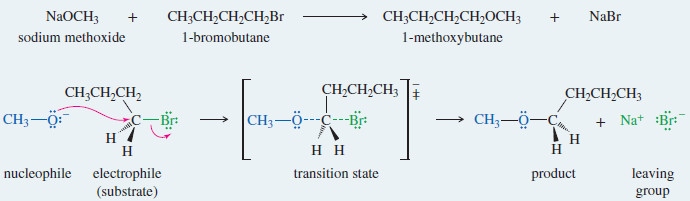
EXAMPLE:
Reaction of 1-bromobutane with sodium methoxide gives 1-methoxybutane
Generality of the SN2 Reaction
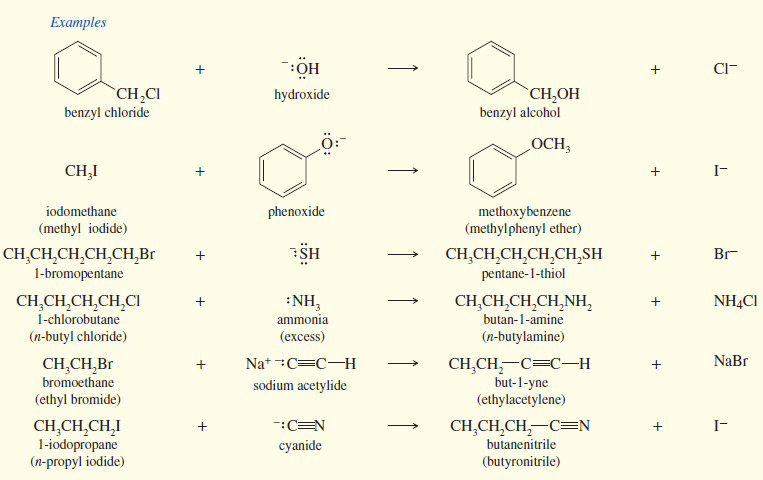
– Many useful reactions take place by SN2 the mechanism.
– The reaction of an alkyl halide, such as methyl iodide, with hydroxide ion gives an alcohol.
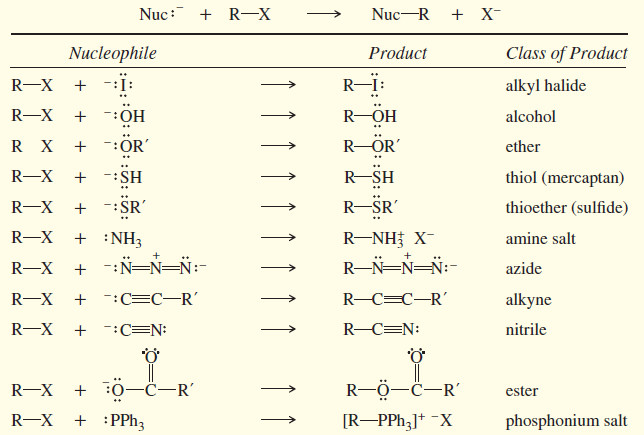
– Other nucleophiles convert alkyl halides to a wide variety of functional groups.
– The following table summarizes some of the types of compounds that can be formed by nucleophilic displacement of alkyl halides
SUMMARY: SN2 Reactions of Alkyl Halides
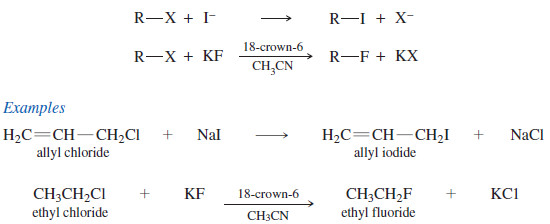
Halogen Exchange Reactions
– The SN2 reaction provides a useful method for synthesizing alkyl iodides and fluorides, which are more difficult to make than alkyl chlorides and bromides.
– Halides can be converted to other halides by halogen exchange reactions, in which one halide displaces another.
– Iodide is a good nucleophile, and many alkyl chlorides react with sodium iodide to give alkyl iodides.
– Alkyl fluorides are difficult to synthesize directly, and they are often made by treating alkyl chlorides or bromides with KF under conditions that use a crown ether to dissolve the fluoride salt in an aprotic solvent, which enhances the normally weak nucleophilicity of the fluoride ion.