E2 Reaction : Second-Order Elimination
Second-Order Elimination: The E2 Reaction
– Eliminations can also take place under second-order conditions with a strong base present.
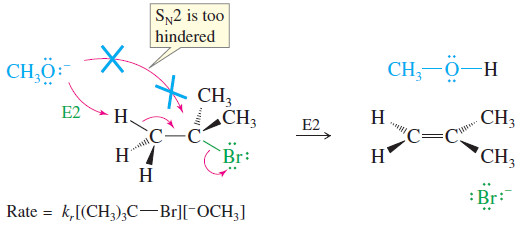
– As an example, consider the reaction of tert-butyl bromide with methoxide ion in methanol.
– This is a second-order reaction because methoxide ion is a strong base as well as a strong nucleophile.
– It attacks the alkyl halide faster than the halide can ionize to give a first-order reaction.
– No substitution product (methyl tert-butyl ether) is observed, however.
– The SN2 mechanism is blocked because the tertiary alkyl halide is too hindered.
– The observed product is 2-methylpropene, resulting from elimination of HBr and formation of a double bond.
– The rate of this elimination is proportional to the concentrations of both the alkyl halide and the base, giving a second-order rate equation.
– This is a bimolecular process, with both the base and the alkyl halide participating in the transition state, so this mechanism is abbreviated E2 for Elimination, bimolecular.
E2 rate = kr [RX] [B:–]
– In the E2 reaction just shown, methoxide reacts as a base rather than as a nucleophile.
– Most strong nucleophiles are also strong bases, and elimination commonly results when a strong base nucleophile is used with a poor SN2 substrate such as a 3° or hindered 2° alkyl halide.
– Instead of attacking the back side of the hindered electrophilic carbon, methoxide abstracts a proton from one of the methyl groups.
– This reaction takes place in one step, with bromide leaving as the base abstracts a proton.
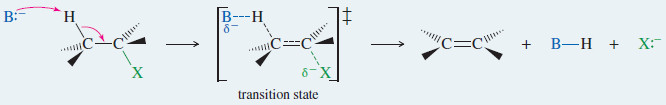
– In the general mechanism of the E2 reaction, a strong base abstracts a proton on a carbon atom adjacent to the one with the leaving group.
– As the base abstracts a proton, a double bond forms and the leaving group leaves.
– Like the SN2 reaction, the E2 is a concerted reaction in which bonds break and new bonds form at the same time, in a single step.
Mechanism: The E2 Reaction
– The concerted E2 reaction takes place in a single step.
– A strong base abstracts a proton on a carbon next to the leaving group, and the leaving group leaves. The product is an alkene
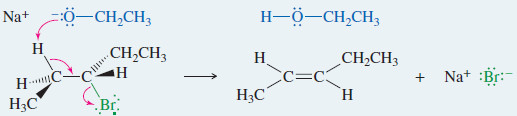
EXAMPLE: E2 elimination of 3-bromopentane with sodium ethoxide.
– The order of reactivity for alkyl halides in E2 reactions is:
3°> 2° > 1°
Reactivity of the Substrate in the E2
– The order of reactivity of alkyl halides toward E2 dehydrohalogenation is found to be:
3° > 2° > 1°
– This reactivity order reflects the greater stability of highly substituted double bonds.
– Elimination of a tertiary halide gives a more substituted alkene than elimination of a secondary halide, which gives a more substituted alkene than a primary halide.
– The stabilities of the alkene products are reflected in the transition states, giving lower activation energies and higher rates for elimination of alkyl halides that lead to highly substituted alkenes.
Mixtures of Products in the E2
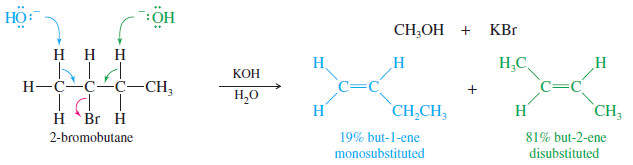
– The E2 reaction requires abstraction of a proton on a carbon atom next to the carbon bearing the halogen.
– If there are two or more possibilities,mixtures of products may result.
– In most cases, Zaitsev’s rule predicts which of the possible products will be the major product: the most substituted alkene.
– For example, the E2 reaction of 2-bromobutane with potassium hydroxide gives a mixture of two products, but-1-ene (a monosubstituted alkene) and but-2-ene (a disubstituted alkene).
– As predicted by Zaitsev’s rule, the disubstituted isomer but-2-ene is the major product.
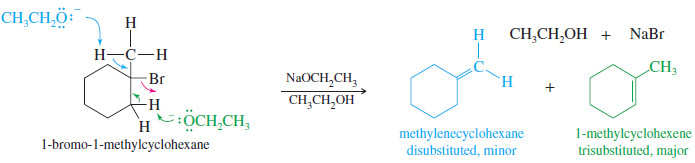
– Similarly, the reaction of 1-bromo-1-methylcyclohexane with sodium ethoxide gives a mixture of a disubstituted alkene and a trisubstituted alkene.
– The trisubstituted alkene is the major product.