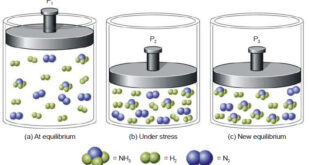
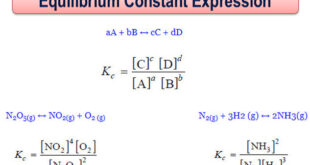Le Chatelier’s principle – In 1884, the French Chemist Henry Le Chatelier proposed a general principle which applies to all systems in equilibrium. This important principle called the Le Chatelier’s principle may be stated as : when a stress is applied on a system in equilibrium, the system tends to …
Read More »Additional Methods for Oxidizing Alcohols
Additional Methods for Oxidizing Alcohols – Many other reagents and procedures have been developed for oxidizing alcohols. – Some are simply modifications of the procedures we have seen. – For example, the Collins reagent is a complex of chromium trioxide and pyridine, the original version of PCC. – The Jones …
Read More »Calculation of Kc from Experimental Information
Calculation of Kc from Experimental Information – To determine the value of Kc of a reaction, write the balanced equation. Then write the equilibrium constant expression. – Substitute in it the equilibrium concentrations of the reactants and products found experimentally. Thus calculate the value of Kc. Solved problem (1) for …
Read More »Oxidation of Alcohols
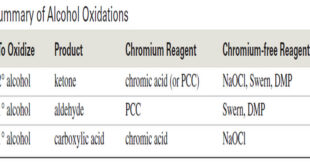
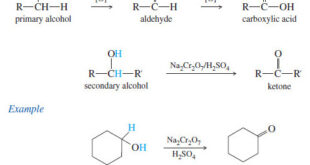
Oxidation of Alcohols – Primary and secondary alcohols are easily oxidized (Oxidation of Alcohols) by a variety of reagents, including chromium oxides, permanganate, nitric acid, and even household bleach (NaOCl, sodium hypochlorite). – The choice of reagent depends on the amount and value of the alcohol. – We use cheap …
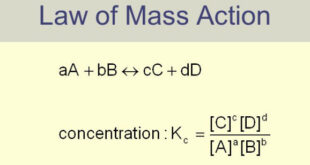
Read More »Equilibrium Constant Expression
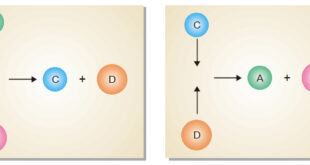
Equilibrium constant: Equilibrium law – Now we will find the Expression of Equilibrium Constant. – Let us consider a general reaction A + B ↔ C + D – and let [A], [B], [C] and [D] represent the molar concentrations of A, B, C and D at the equilibrium point. …
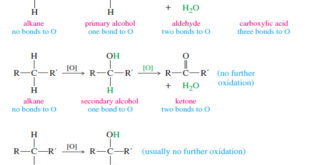
Read More »Oxidation states of Alcohols and Related Functional Groups
Oxidation states of Alcohols and Related Functional Groups – Oxidation states of Alcohols leads to ketones, aldehydes, and carboxylic acids. – These functional groups, in turn, undergo a wide variety of additional reactions. – For these reasons, alcohol oxidations are some of the most common organic reactions. – In inorganic …
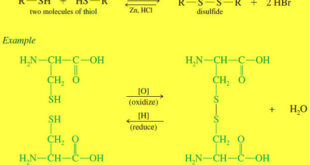
Read More »Thiols (Mercaptans)
What is Thiols? – Thiols are sulfur analogues of alcohols, with an -SH group in place of the alcohol -OH group. – Oxygen and sulfur are in the same column of the periodic table (group 6A), with oxygen in the second row and sulfur in the third. Nomenclature of Thiols …
Read More »Law of Mass action
Law of Mass action – Two Norwegian chemists, Guldberg and Waage, studied experimentally a large number of equilibrium reactions. In 1864, they postulated a generalisation called the Law of Mass action. – Law of Mass action states that : the rate of a chemical reaction is proportional to the active …
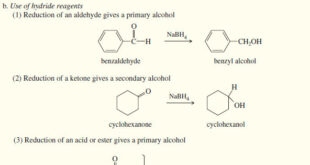
Read More »Reduction of the Carbonyl group : Synthesis of Alcohols
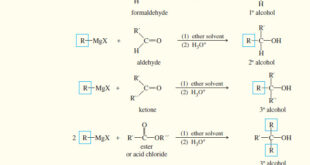
Reduction of the Carbonyl group : Synthesis of 1° and 2° Alcohols – Grignard reagents convert carbonyl group to alcohols by adding alkyl groups. – Hydride reagents add a hydride ion (H:–) reducing the carbonyl group to an alkoxide ion with no additional carbon atoms. – Subsequent protonation gives the …
Read More »Characteristics of Chemical Equilibrium
Chemical Equilibrium is the state of a reversible reaction when the two opposing reactions occur at the same rate and the concentrations of reactants and products do not change with time. Nature of Chemical Equilibrium : its definition – Let us consider the reaction A + B ↔ C + …
Read More »Side Reactions of Organometallic Reagents
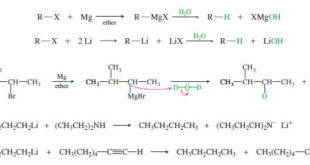
Side Reactions of Organometallic Reagents: Reduction of Alkyl Halides – Organometallic Reagents: Grignard and organolithium reagents are strong nucleophiles and strong bases. – Besides their additions to carbonyl compounds, they react with other acidic or electrophilic compounds. – In some cases, these are useful reactions, but they are often seen …
Read More »Addition of Grignard Reagents to Carbonyl Compounds
Addition of Organometallic Reagents to Carbonyl Compounds – Because they resemble carbanions, Grignard reagents and organolithium reagents are strong nucleophiles and strong bases. – Their most useful nucleophilic reactions are additions to carbonyl (C=O) groups, much like we saw with acetylide ions – The carbonyl group is polarized, with a …
Read More »Organometallic Reagents for Alcohol Synthesis
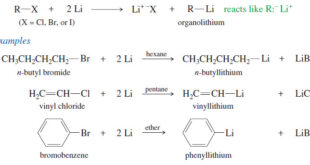
Organometallic Reagents for Alcohol Synthesis – Organometallic compounds contain covalent bonds between carbon atoms and metal atoms. – And Organometallic reagents are useful because they have nucleophilic carbon atoms, in contrast to the electrophilic carbon atoms of alkyl halides. – Most metals (M) are more electropositive than carbon, and the …
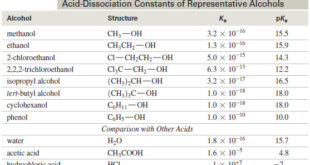
Read More »Acidity of Alcohols and Phenols
Acidity of Alcohols and Phenols – we will talk here about some Acidity of Alcohols and Phenols. – Like the hydroxyl proton of water, the hydroxyl proton of an alcohol is weakly acidic. – A strong base can remove the hydroxyl proton to give an alkoxide ion. – The acidities …
Read More »Commercially Important Alcohols
we will talk about some Commercially Important Alcohols such as: Methanol , Ethanol , isopropyl alcohol (1) Commercially Important Alcohols: Methanol – Methanol (methyl alcohol) was originally produced by the destructive distillation of wood chips in the absence of air. This source led to the name wood alcohol. – During …
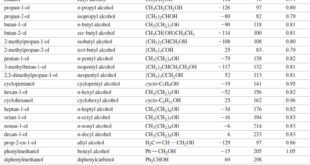
Read More »Physical Properties of Alcohols
We will discuss here Physical Properties of Alcohols: (A) Boiling Points of Alcohols (B) Solubility Properties of Alcohols Physical Properties of Alcohols – Most of the common alcohols, up to about 11 or 12 carbon atoms, are liquids at room temperature. – Methanol and ethanol are free-flowing volatile liquids with …
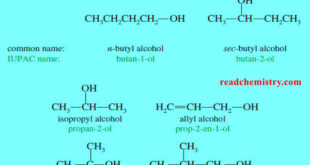
Read More »Nomenclature of Alcohols and Phenols
In this subject we will talk about Nomenclature of Alcohols and Phenols (1) Nomenclature of Alcohols: IUPAC Names – The IUPAC system provides unique names for alcohols, based on rules that are similar to those for other classes of compounds. – In general, the name carries the (-ol) suffix, together …
Read More »First law of thermodynamics – MCQ online test
Online MCQ test on First law of thermodynamics – In this topic we offer you, online MCQ test in the fundmental of First law of thermodynamics. – It is a simple test to measure the strength of your knowledge in the basics of understanding the topics of First law of …
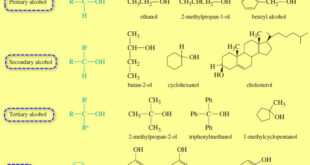
Read More »Structure and Classification of Alcohols
– In this subject we will talk about Structure and Classification of Alcohols. What are Alcohols? – Alcohols are organic compounds containing hydroxyl (-OH) groups. – They are some of the most common and useful compounds in nature, in industry, and around the house. – The word alcohol is one …
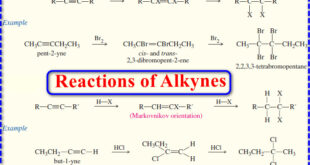
Read More »Reactions of Alkynes
Reactions of Alkynes – Many of the reactions of alkynes are similar to the corresponding reactions of alkenes because both involve pi bonds between two carbon atoms. – Like the pi bond of an alkene, the pi bonds of an alkyne are electron-rich, and they readily undergo addition reactions. (A) …
Read More » Read Chemistry
Read Chemistry