Weighing Equipment and Manipulations
– In this subject, we will discuss The Equipment and Manipulations Associated with weighing.
Weighing Equipment and Manipulations
– The mass of many solids changes with humidity because they tend to absorb weighable amounts of moisture.
– This effect is especially pronounced when a large surface area is exposed, as with a reagent chemical or a sample that has been ground to a fine powder.
– In the first step in a typical analysis, then, the sample is dried so that the results will not be affected by the humidity of the surrounding atmosphere.
– A sample, a precipitate, or a container is brought to constant mass by a cycle of heating (usually for one hour or more) at an appropriate temperature, cooling, and weighing.
– This cycle is repeated as many times as needed to obtain successive masses that agree within 0.2 to 0.3 mg of one another.
– The establishment of constant mass provides some assurance that the chemical or physical processes that occur during the heating (or ignition) are complete.
(1) Weighing Bottles
– Weighing bottles are convenient for drying and storing solids.
– Two common varieties of these handy tools are shown in the Figure below.
– The ground-glass portion of the cap-style bottle shown on the left is on the outside and does not come into contact with the contents.
– This design eliminates the possibility of some of the samples becoming trapped on the ground-glass surface and subsequently being lost.
– Ruggedness is a principal advantage of using plastic weighing bottles rather than glass, but plastic abrades easily and is not as easily cleaned as glass.
(2) Desiccators and Desiccants
– Oven drying is the most common way of removing moisture from solids.
– This approach is not appropriate for substances that decompose or for those from which water is not removed at the temperature of the oven.
– To minimize the uptake of moisture, dried materials are stored in desiccators while they cool.
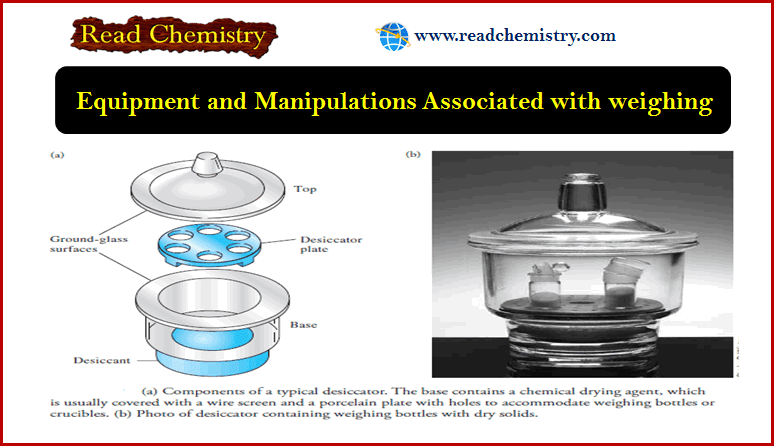
– The figure shows the components of a typical desiccator.
– The base section contains a chemical drying agent, such as anhydrous calcium chloride, calcium sulfate (Drierite), anhydrous magnesium perchlorate (Anhydrone or Dehydrite), or phosphorus pentoxide.
– The ground-glass surfaces between the top and the base are lightly coated with grease to ensure a good seal when the top is in place.
– A desiccator is a device for drying substances or objects.
– When removing or replacing the lid of a desiccator, use a sliding motion to minimize the likelihood of disturbing the sample.
– An airtight seal is achieved by slight rotation and downward pressure on the positioned lid.
– When placing a heated object in a desiccator, the increase in pressure as the enclosed air is warmed may be sufficient to break the seal between the lid and base.
– Conversely, if the seal is not broken, the cooling of heated objects can cause a partial vacuum to develop. Both of these conditions can cause the contents of the desiccator to be physically lost or contaminated.
– Although it defeats the purpose of the desiccator somewhat, allow some cooling to occur before the lid is seated.
– It is also helpful to break the seal once or twice during cooling to relieve any excessive vacuum that develops.
– Finally, lock the lid in place with your thumbs while moving the desiccator from one place to another.
Notes
– Very hygroscopic materials should be stored in containers equipped with snug covers, such as weighing bottles.
– The bottles remain covered while in the desiccator. Most other solids can be safely stored uncovered.
(3) Manipulating Weighing Bottles
– Heating at 105°C to 110°C for 1 hour is sufficient to remove the moisture from the surface of most solids.
– The figure shows the recommended way to dry a sample.
– The weighing bottle is contained in a labeled beaker with a cover glass.
– This arrangement protects the sample from accidental contamination and also allows for free access to air.
– Crucibles containing a precipitate that can be freed of moisture by simple drying can be treated similarly.
– The beaker holding the weighing bottle or crucible to be dried must be carefully marked for identification.
– Avoid touching dried objects with your fingers because detectable amounts of water or oil from the skin may be transferred to the objects.
– Instead, use tongs, chamois finger cots, clean cotton gloves, or strips of paper to handle dried objects for weighing.
– The figure shows how a weighing bottle is manipulated with tongs and strips of paper.
(4) Weighing by Difference
– Weighing by difference is a simple method for determining a series of sample masses.
– First, the bottle and its contents are weighed.
– One sample is then transferred from the bottle to a container.
– The gentle tapping of the bottle with its top and slight rotation of the bottle provides control over the amount of sample removed.
– Following the transfer, the bottle, and its residual contents are weighed.
– The mass of the sample is the difference between the two masses.
– It is essential that all the solids removed from the weighing bottle be transferred without loss to the container.
(5) Weighing Hygroscopic Solids
– Hygroscopic substances rapidly absorb moisture from the atmosphere and, therefore, require special handling.
– You need a weighing bottle for each sample to be weighed.
– Place the approximate amount of sample needed in the individual bottles and heat for an appropriate time.
– When heating is complete, quickly cap the bottles and cool them in a desiccator.
– Weigh one of the bottles after opening it momentarily to relieve any vacuum.
– Quickly empty the contents of the bottle into its receiving vessel, cap immediately, and weigh the bottle again along with any solid that did not get transferred.
– Repeat for each sample and determine the sample masses by difference.
(6) Weighing Liquids
– The mass of a liquid is always obtained by difference.
– Liquids that are noncorrosive and relatively nonvolatile can be transferred to previously weighed containers with snugly fitting covers (such as weighing bottles).
– The mass of the container is subtracted from the total mass.
– A volatile or corrosive liquid should be sealed in a weighed glass ampoule.
– The ampoule is heated, and the neck is then immersed in the sample.
– As cooling occurs, the liquid is drawn into the bulb.
– The ampoule is then inverted and the neck is sealed off with a small flame.
– The ampoule and its contents, along with any glass removed during sealing, are cooled to room temperature and weighed.
– Thus, the ampoule is then transferred to an appropriate container and broken.
– A volume correction for the glass of the ampoule may be needed if the receiving vessel is a volumetric flask.
Reference: Fundamentals of analytical chemistry / Douglas A. Skoog, Donald M. West, F. James Holler, Stanley R. Crouch. (ninth edition), 2014. USA