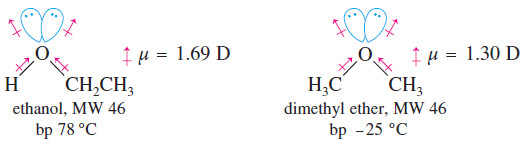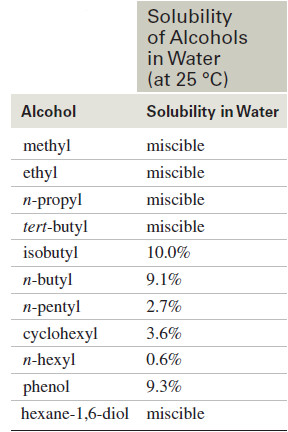Physical Properties of Alcohols
We will discuss here Physical Properties of Alcohols:
(A) Boiling Points of Alcohols
(B) Solubility Properties of Alcohols
Physical Properties of Alcohols
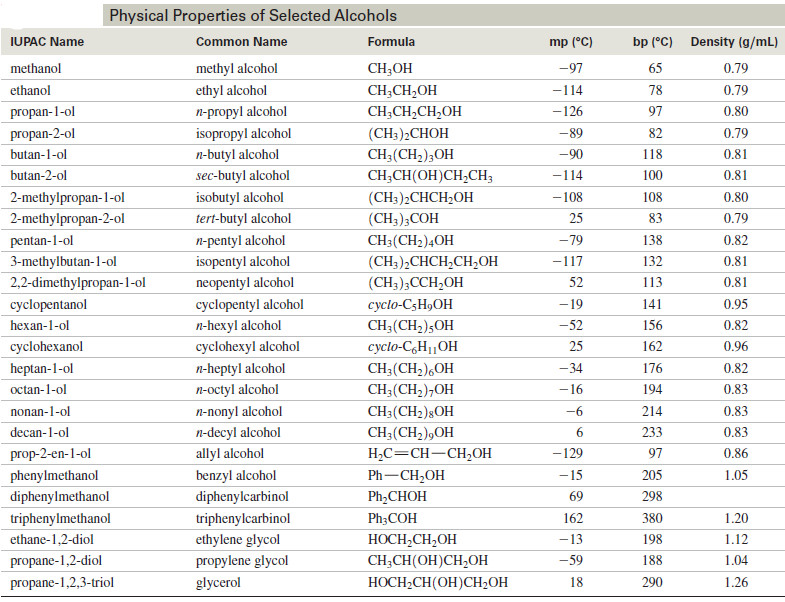
– Most of the common alcohols, up to about 11 or 12 carbon atoms, are liquids at room temperature.
– Methanol and ethanol are free-flowing volatile liquids with characteristic fruity odors.
– The higher alcohols (the butanols through the decanols) are somewhat viscous, and some of the highly branched isomers are solids at room temperature.
– These higher alcohols have heavier but still fruity odors.
– Propan-1-ol and propan-2-ol fall in the middle, with a barely noticeable viscosity and a characteristic odor often associated with a physician’s office.
– The following lists the physical properties of some common alcohols
(A) Boiling Points of Alcohols
– Because we often deal with liquid alcohols, we forget how surprising it should be that the lower-molecular-weight alcohols are liquids.
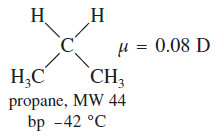
– For example, ethyl alcohol and propane have similar molecular weights, yet their boiling points differ by about 120 °C.
– Dimethyl ether has an intermediate boiling point
– Such a large difference in boiling points suggests that ethanol molecules are attracted to each other much more strongly than propane molecules.
– Two important intermolecular forces are responsible: hydrogen bonding and dipole–dipole attractions.
Hydrogen Bonds
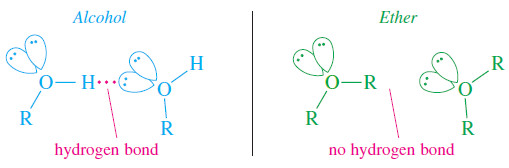
– Hydrogen bonding is the major intermolecular attraction responsible for ethanol’s high boiling point.
– The hydroxyl hydrogen of ethanol is strongly polarized by its bond to oxygen, and it forms a hydrogen bond with a pair of nonbonding electrons from the oxygen atom of another alcohol molecule.
– Ethers have two alkyl groups bonded to their oxygen atoms, so they have no O¬H hydrogen atoms to form hydrogen bonds.
Hydrogen bonds have a strength of about 21 kJ (5 kcal) per mole: weaker than typical covalent bonds of 300 to 500 kJ, but much stronger than dipole–dipole attractions.
Dipole–Dipole attractions
– Dipole–dipole attractions also contribute to the relatively high boiling points of alcohols and ethers.
– The polarized C-O and H-O bonds and the nonbonding electrons add to produce a dipole moment of 1.69 D in ethanol, compared with a dipole moment of only 0.08 D in propane.
– In liquid ethanol, the positive and negative ends of these dipoles align to produce attractive interactions.
– We can compare the effects of hydrogen bonding and dipole–dipole attractions by comparing ethanol with dimethyl ether.
– Like ethanol, dimethyl ether has a large dipole moment (1.30 D), but dimethyl ether cannot engage in hydrogen bonding because it has no -O-H hydrogens
The boiling point of dimethyl ether is -25 °C about 17° higher than that of propane, but still 103° lower than that of ethanol.
– Hydrogen bonds are clearly much stronger intermolecular attractions than dipole–dipole attractions.
(B) Solubility Properties of Alcohols
– Water and alcohols have similar properties because they all contain hydroxyl groups that can form hydrogen bonds.
– Alcohols form hydrogen bonds with water, and several of the lower-molecular-weight alcohols are miscible (soluble in any proportions) with water.
– Similarly, alcohols are much better solvents than hydrocarbons for polar substances.
– Significant amounts of ionic compounds such as sodium chloride can dissolve in some of the lower alcohols.
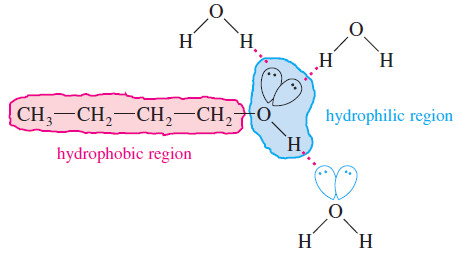
– We call the hydroxyl group hydrophilic, meaning (water loving,) because of its affinity for water and other polar substances.
– The alcohol’s alkyl group is called hydrophobic (water hating) because it acts like an alkane: It disrupts the network of hydrogen bonds and dipole–dipole attractions of a polar solvent such as water.
– The alkyl group makes the alcohol less hydrophilic, yet it lends solubility in nonpolar organic solvents.
– As a result, most alcohols are miscible with a wide range of nonpolar organic solvents.
The following lists the solubility of some simple alcohols in water.
– The water solubility decreases as the alkyl group becomes larger.
– Alcohols with one-, two-, or three-carbon alkyl groups are miscible with water.
– A four-carbon alkyl group is large enough that some isomers are not miscible, yet tert-butyl alcohol, with a compact spherical shape, is miscible.
– In general, each hydroxyl group or other hydrogen-bonding group can carry about four carbon atoms into water.
– Hexan-1-ol, with six carbon atoms, is only slightly soluble in water, but hexane-1,6-diol, with two hydrogen-bonding groups, is miscible with water.
– Phenol is unusually soluble for a six-carbon alcohol because of its compact shape and the particularly strong hydrogen bonds formed between phenolic -OH groups and water molecules