** The (s) and (p) orbitals used in the quantum mechanical description of the carbon atom, were based on calculations for hydrogen atoms. These simple (s) and (p) orbitals do not, when taken alone, provide a satisfactory model for the tetravalent–tetrahedral carbon of methane. ** However, a satisfactory …
Read More »Resonance Theory
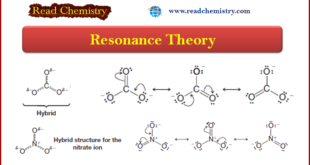
Introduction to Resonance (Resonance in carbonate ion (CO32-) ** Often more than one equivalent Lewis structure can be written for a molecule or ion. Consider, for example, the carbonate ion (CO32-). We can write three different but equivalent structures, 1–3: ** Notice two important features of these …
Read More »Percent Composition of Compounds
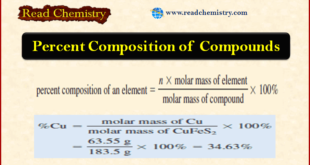
Percent Composition of Compounds **As we have seen, the formula of a compound tells us the numbers of atoms of each element in a unit of the compound. ** However, suppose we needed to verify the purity of a compound for use in a laboratory experiment. We could calculate …
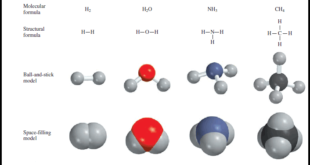
Read More »How to write and interpret Structural Formulas?
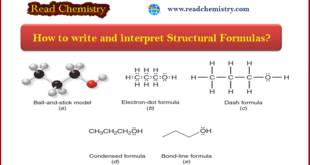
** Organic chemists use a variety of formats to write structural formulas. ** Structural Formulas are written by: (1) Ball-and-stick model (2) Electron-dot formula (lewis structure) (3) Dash formula (4) Condensed formula (5) Bond-line formula ** Examples of these types of structural formulas are shown in the …
Read More »Radioactive decay: Definition, Types, Examples
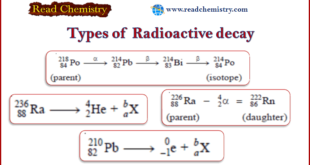
– In this subject, we will discuss the Radioactive decay: Definition, Types, Examples Types of Radioactive Decay – According to the theory put forward by Rutherford and Soddy (1903), radioactivity is a nuclear property. – The nucleus of a radioactive atom is unstable. – It undergoes decay or disintegration by …
Read More »Radioactivity: Detection and Measurement
– In this subject, we will discuss the Detection and measurement of Radioactivity. Detection and measurement of Radioactivity – Radioactivity can be detected and measured by several methods. – The important ones used in modern practice are listed below. (1) Cloud Chamber (2) Ionization Chamber (3) Geiger-Muller Counter (4) Scintillation …
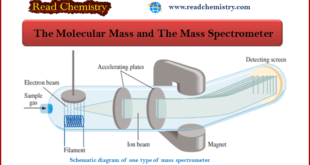
Read More »The Molecular Mass and The Mass Spectrometer
Molecular Mass ** If we know the atomic masses of the component atoms, we can calculate the mass of a molecule. ** The molecular mass: (sometimes called molecular weight) is the sum of the atomic masses (in amu) in the molecule. ** For example, the molecular mass of …
Read More »Avogadro’s Number and the Molar Mass of an Element
Avogadro’s Number (NA) ** Atomic mass units provide a relative scale for the masses of the elements. ** But because atoms have such small masses, no usable scale can be devised to weigh them in calibrated units of atomic mass units. ** In any real situation, we deal with macroscopic …
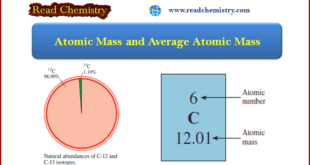
Read More »Atomic Mass and Average Atomic Mass: Definition, Calculation
– In this subject, we will discuss the Atomic Mass and Average Atomic Mass: Definition, Formula, and Calculation Atomic Mass – The mass of an atom depends on the number of electrons, protons, and neutrons it contains. – Knowledge of an atom’s mass is important in laboratory work. – But …
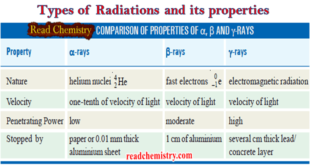
Read More »Types of Radiations and its properties
Nuclear reaction ** A nuclear reaction is different from a chemical reaction. ** In a chemical reaction, atoms of the reactants combine by a rearrangement of extranuclear electrons but the nuclei of the atoms remain unchanged. ** In a nuclear reaction, on the other hand, it is the …
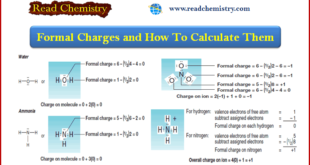
Read More »Formal Charge: Definition, Formula, Calculation, Examples
– In this subject, we will discuss the Formal Charge: Definition, Formula, Calculation, Examples Formal Charge and How To Calculate – Many Lewis structures are incomplete until we decide whether any of their atoms have a formal charge. – Calculating the formal charge on an atom in a Lewis structure …
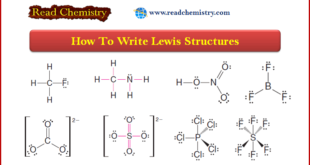
Read More »Lewis Structures: Definition, Structural Formula, Examples
– In this subject, we will discuss the Lewis Structures: Definition, Overview, Structural Formula, Examples Definition of Lewis structures – A Lewis structure is a structural representation of a molecule where dots are used to show electron positions around the atoms and lines or dot pairs represent covalent bonds between …
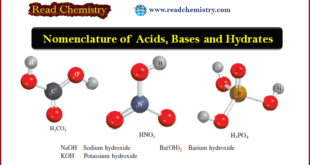
Read More »Nomenclature of Acids, Bases and Hydrates
– In this subject, we will discuss the Nomenclature of Acids, Bases and Hydrates Nomenclature of Acids – An acid can be described as a substance that yields hydrogen ions (H+) when dissolved in water. (H+ is equivalent to one proton, and is often referred to that way.) – Formulas …
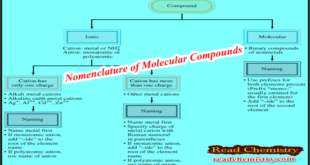
Read More »Nomenclature of Molecular Compounds
How to Name Molecular Compounds – Unlike ionic compounds, Molecular compounds contain discrete molecular units. – They are usually composed of nonmetallic elements. – Many molecular compounds are binary compounds. – Naming binary molecular compounds is similar to naming binary ionic compounds. – We place the name of the first …
Read More »Nomenclature of Ionic Compounds
– In this subject, we will discuss the Nomenclature of Ionic Compounds. Naming Compounds – In addition to using formulas to show the composition of molecules and compounds, chemists have developed a system for naming substances based on their composition. – we divided naming substances into three categories: (1) Nomenclature of …
Read More »Molecular Formulas and Empirical Formulas: Definitions, Examples
– In this subject, we will discuss Molecular Formulas and Empirical Formulas: Definitions, Examples – Chemists use Chemical formulas to express the composition of molecules and ionic compounds in terms of chemical symbols. – By composition we mean not only the elements present but also the ratios in which the atoms …
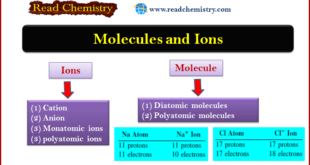
Read More »Atoms, Molecules, and Ions: Definition, Types, Examples
– In this subject, we will discuss the Atoms, Molecules, and Ions: Definition, Types, Examples Atoms – Of all the elements, only the six noble gases in Group 8A of the periodic table (He, Ne, Ar, Kr, Xe, and Rn) exist in nature as single atoms. – For this reason, …
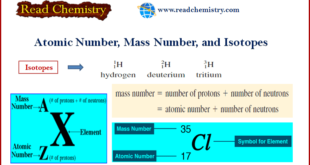
Read More »Atomic Number, Mass Number, and Isotopes
– In this subject, we will discuss the Atomic Number, Mass Number, and Isotopes: Definition, Formula, and Calculation Atomic number – All atoms can be identified by the number of protons and neutrons they contain. – The number of protons in the nucleus of each atom of an element is …
Read More »MCQ on: Isotopes, Isobars, and Isotones
MCQ on: Isotopes, Isobars, and Isotones – In this subject, you will find 25 questions and answers MCQ on Isotopes, Isobars, and Isotones. 1. The atoms of an element that have the same number of protons and different numbers of neutrons are called_______ (a) isotopes (b) isobars (c) isotones (d) …
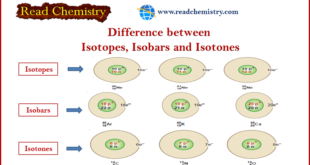
Read More »Difference between Isotopes, Isobar, and Isotones
In this subject, the Difference between Isotopes, Isobar, and Isotones will be discussed with some Common Examples for all types. What are Isotopes? Isotopes may be defined as: (1) The atoms of an element that have the same number of protons and different number of neutrons are called Isotopes. (2) …
Read More » Read Chemistry
Read Chemistry