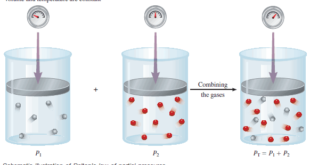
– In this subject, we will discuss Dalton’s Law of Partial Pressures (Statement, Mathematical, Importance, Application). Dalton’s Law of Partial Pressures – Thus far we have concentrated on the behavior of pure gaseous substances, but experimental studies very often involve mixtures of gases. – For example, for a study of air …
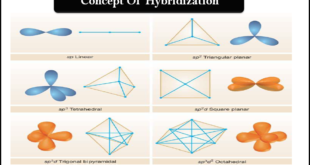
Read More »Hybridization and Shapes of Molecules
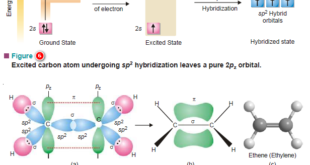
Hybridization and Shapes of Molecules – In the previous subject, we talked about the concept of Hybridization and the types of Hybridization, but in this subject, we will talk about Hybridization and Shapes of Molecules. – Diatomic molecules must all be invariably linear but tri-and tetra-atomic molecules have several possible …
Read More »Density of gas: Definition, Equation, Solved Examples
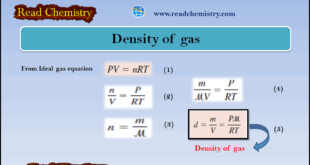
– In this subject, we will discuss the Density of gas (Definition, Equation, Solved Examples) The Density of gas – From the ideal gas equation: – If we rearrange the ideal gas equation, we can calculate the density of a gas: – The number of moles of the gas, n, …
Read More »Molar Mass of gas: Definition, Equation, Solved Examples
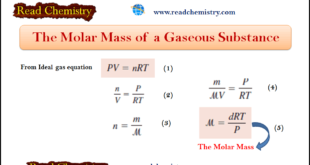
– In this subject, we will discuss the Molar Mass of gas (Definition, Equation, Solved Examples) The Molar Mass of a gas – From what we have seen so far, you may have the impression that the molar mass of a substance is found by examining its formula and summing …
Read More »Hybridization: Definition, Types, Rules, Examples
– In this subject, we will discuss the Hybridization: Definition, Types, Rules, and Examples – While the formation of simple molecules could be explained adequately by the overlap of atomic orbitals, the formation of molecules of Be, B, and C present problems of greater magnitude having no solution with the …
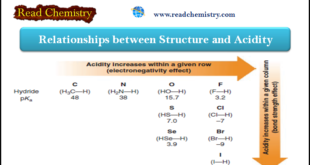
Read More »Acidity: The relationship between Structure and Acidity
– In this subject, we will discuss the Relationships between Structure and Acidity. – The strength of a Brønsted–Lowry acid depends on the extent to which a proton can be separated from it and transferred to a base. – Removing the proton involves breaking a bond to the proton, and …
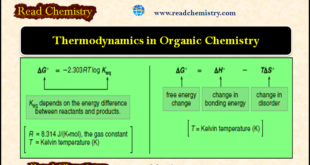
Read More »Thermodynamics of Organic Compounds
– In this subject, we will discuss the Thermodynamics of Organic Compounds Thermodynamics of Organic Compounds – For a reaction to be practical, the equilibrium must favor the products, and the reaction rate must be fast enough to form them in a reasonable time. – These two conditions depend on …
Read More »How to Predict the Outcome of acid-base reaction
– In this subject, we will discuss How to Predict the Outcome of acid-base reaction. How To Predict the Outcome of acid-base reaction – The following table gives the approximate pKa values for a range of representative compounds. – While you probably will not be expected to memorize all of the …
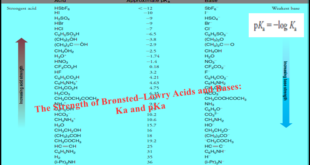
Read More »Bronsted-Lowry Acid Strength: Ka and pKa
– In this subject, we will discuss the Bronsted-Lowry Acid Strength: Ka and pKa Bronsted-Lowry Acid Strength: Ka and pKa – Many organic reactions involve the transfer of a proton by an acid–base reaction. – An important consideration, therefore, is the relative strengths of compounds that could potentially act as …
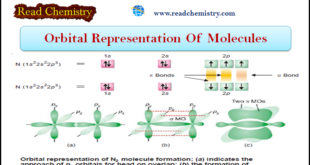
Read More »Orbital Representation of Molecules
– In this subject, we will discuss the Orbital Representation of some Molecules (1) Orbital Representation of H2 molecule – Each hydrogen atom has one electron in 1s-orbital. – Two such atoms join to form a molecule of hydrogen. – In this case s–s overlapping between two 1s-orbitals of hydrogen …
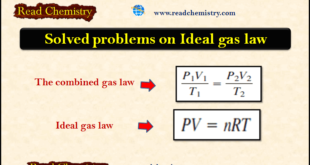
Read More »The Ideal gas law: Solved Problems
– In this subject, we will discuss Solved Problems on The Ideal gas law Problem (1) on The ideal gas law Calculate the volume of a sample of gas originally occupying 908 mL at 717 torr and 20 oC after its temperature and pressure are changed to 72 oC and …
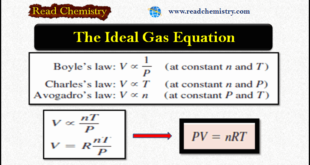
Read More »Ideal Gas Equation: Definition, Formula, Notes
– In this subject, we will discuss the Ideal Gas Equation (Definition, Formula, Notes) The Ideal Gas Equation – Let us summarize the gas laws we have discussed so far: – We can combine all three expressions to form a single master equation for the behavior of gases: – This …
Read More »Physical Chemistry book , 3rd edition by Robert G. Mortimer
– In this subject, we will discuss free download of Physical Chemistry book, 3rd edition by Robert G. Mortimer The Preface of Physical Chemistry book – The book is divided into four parts: – The first part focuses on the macroscopic properties of physical systems. It begins with the …
Read More »Fundamentals of Electrochemistry book by V.S. Bagotsky
– In this subject, we will discuss free download of Fundamentals of Electrochemistry book by V.S. Bagotsky The Preface of Fundamentals of Electrochemistry book – Two very important fields of natural science—chemistry and the science of electricity— matured and grew vigorously during the first half of the nineteenth century. Electrochemistry …
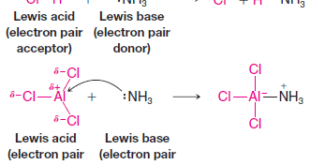
Read More »Lewis Acids and Lewis Bases
– In this subject, we will discuss the Lewis acid-base theory and Lewis Acids and Lewis Bases Lewis Acids and Lewis Bases – In 1923 G. N. Lewis proposed a theory that significantly broadened the understanding of acids and bases. – As we go along we shall find that an …
Read More »Avogadro’s Law: The Volume-Amount Relationship
– In this subject, we will discuss the Avogadro’s Law: The Volume-Amount Relationship Avogadro’s Law: The Volume-Amount Relationship – The work of the Italian scientist Amedeo Avogadro complemented the studies of Boyle, Charles, and Gay-Lussac. – In 1811 Avogadro published a hypothesis stating that at the same temperature and pressure, …
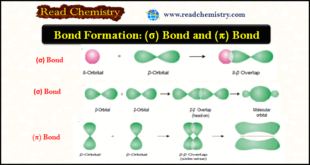
Read More »Bond Formation, (σ) Bond and (π) Bond
Bond Formation (Valence Bond Theory) – Bond formation between atoms to give chemical compounds can be interpreted admirably in terms of the orbital theory of atomic structure. – Heitler and London believed that electron cloud of the valence orbital on one atom ‘overlaps’ the electron cloud of the other bonding …
Read More »Charles’s Law: Relationship Between Temperature And Volume
– In this subject, we will discuss Charles’s Law: Relationship Between Temperature And Volume ( V-T relationship). Relationship Between Temperature And Volume – Boyle’s law depends on the temperature of the system remaining constant. – But suppose the temperature changes: How does a temperature change affect the volume and pressure …
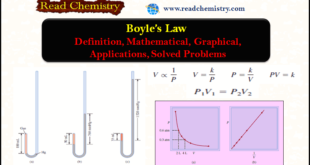
Read More »Boyle’s Law: Definition, Mathematical, Graphical, Applications
– In this subject, we will discuss Boyle’s Law: Definition, Mathematical, Graphical, Applications How did Boyle discover his Law? – Robert Boyle (1627–1691) is a British chemist and natural philosopher. – Although Boyle is commonly associated with the gas law that bears his name, he made many other significant contributions …
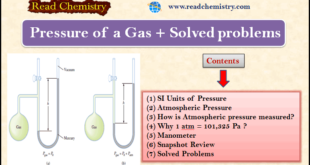
Read More »Gas Pressure: Definition, Formula and Solved problems
– In this subject, we will discuss Gas Pressure: Definition, Formula, and Solved problems Gas Pressure – Gases exert pressure on any surface with which they come in contact because gas molecules are constantly in motion. – We, humans, have adapted so well physiologically to the pressure of the air …
Read More » Read Chemistry
Read Chemistry