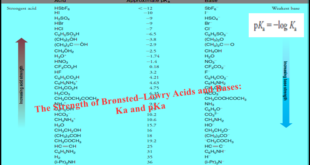
– In this subject, we will discuss How to Predict the Outcome of acid-base reaction. How To Predict the Outcome of acid-base reaction – The following table gives the approximate pKa values for a range of representative compounds. – While you probably will not be expected to memorize all of the …
Read More »Bronsted-Lowry Acid Strength: Ka and pKa
– In this subject, we will discuss the Bronsted-Lowry Acid Strength: Ka and pKa Bronsted-Lowry Acid Strength: Ka and pKa – Many organic reactions involve the transfer of a proton by an acid–base reaction. – An important consideration, therefore, is the relative strengths of compounds that could potentially act as …
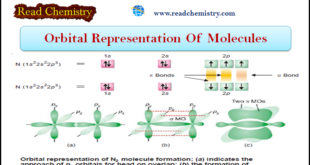
Read More »Orbital Representation of Molecules
– In this subject, we will discuss the Orbital Representation of some Molecules (1) Orbital Representation of H2 molecule – Each hydrogen atom has one electron in 1s-orbital. – Two such atoms join to form a molecule of hydrogen. – In this case s–s overlapping between two 1s-orbitals of hydrogen …
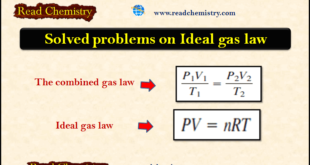
Read More »The Ideal gas law: Solved Problems
– In this subject, we will discuss Solved Problems on The Ideal gas law Problem (1) on The ideal gas law Calculate the volume of a sample of gas originally occupying 908 mL at 717 torr and 20 oC after its temperature and pressure are changed to 72 oC and …
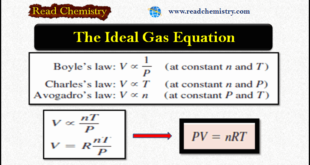
Read More »Ideal Gas Equation: Definition, Formula, Notes
– In this subject, we will discuss the Ideal Gas Equation (Definition, Formula, Notes) The Ideal Gas Equation – Let us summarize the gas laws we have discussed so far: – We can combine all three expressions to form a single master equation for the behavior of gases: – This …
Read More »Physical Chemistry book , 3rd edition by Robert G. Mortimer
– In this subject, we will discuss free download of Physical Chemistry book, 3rd edition by Robert G. Mortimer The Preface of Physical Chemistry book – The book is divided into four parts: – The first part focuses on the macroscopic properties of physical systems. It begins with the …
Read More »Fundamentals of Electrochemistry book by V.S. Bagotsky
– In this subject, we will discuss free download of Fundamentals of Electrochemistry book by V.S. Bagotsky The Preface of Fundamentals of Electrochemistry book – Two very important fields of natural science—chemistry and the science of electricity— matured and grew vigorously during the first half of the nineteenth century. Electrochemistry …
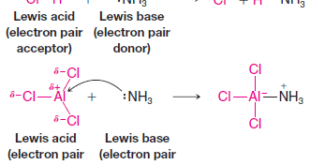
Read More »Lewis Acids and Lewis Bases
– In this subject, we will discuss the Lewis acid-base theory and Lewis Acids and Lewis Bases Lewis Acids and Lewis Bases – In 1923 G. N. Lewis proposed a theory that significantly broadened the understanding of acids and bases. – As we go along we shall find that an …
Read More »Avogadro’s Law: The Volume-Amount Relationship
– In this subject, we will discuss the Avogadro’s Law: The Volume-Amount Relationship Avogadro’s Law: The Volume-Amount Relationship – The work of the Italian scientist Amedeo Avogadro complemented the studies of Boyle, Charles, and Gay-Lussac. – In 1811 Avogadro published a hypothesis stating that at the same temperature and pressure, …
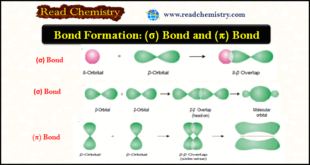
Read More »Bond Formation, (σ) Bond and (π) Bond
Bond Formation (Valence Bond Theory) – Bond formation between atoms to give chemical compounds can be interpreted admirably in terms of the orbital theory of atomic structure. – Heitler and London believed that electron cloud of the valence orbital on one atom ‘overlaps’ the electron cloud of the other bonding …
Read More »Charles’s Law: Relationship Between Temperature And Volume
– In this subject, we will discuss Charles’s Law: Relationship Between Temperature And Volume ( V-T relationship). Relationship Between Temperature And Volume – Boyle’s law depends on the temperature of the system remaining constant. – But suppose the temperature changes: How does a temperature change affect the volume and pressure …
Read More »Boyle’s Law: Definition, Mathematical, Graphical, Applications
– In this subject, we will discuss Boyle’s Law: Definition, Mathematical, Graphical, Applications How did Boyle discover his Law? – Robert Boyle (1627–1691) is a British chemist and natural philosopher. – Although Boyle is commonly associated with the gas law that bears his name, he made many other significant contributions …
Read More »Gas Pressure: Definition, Formula and Solved problems
– In this subject, we will discuss Gas Pressure: Definition, Formula, and Solved problems Gas Pressure – Gases exert pressure on any surface with which they come in contact because gas molecules are constantly in motion. – We, humans, have adapted so well physiologically to the pressure of the air …
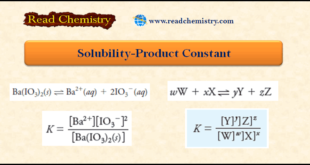
Read More »Solubility Product Ksp: Definition, Formula, Problems
– In this subject, we will discuss the Solubility Product (Ksp): Definition, Formula, Problems Solubility Product Constant Ksp – Most, but not all, sparingly soluble salts are essentially completely dissociated in a saturated aqueous solution. – For example, when an excess of barium iodate is equilibrated with water, the dissociation …
Read More »Gaseous Substances: Substances That Exist as Gases
– In this subject, we will discuss the Gaseous Substances: Substances That Exist as Gases Gaseous Substances – Under certain conditions of pressure and temperature, most substances can exist in any one of three states of matter: solid, liquid, or gas. – Water, for example, can be solid ice, liquid …
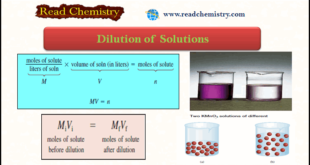
Read More »Dilution of Solutions
– In this subject, we will discuss the Dilution of Solutions and Dilution Law. Dilution of Solutions – Concentrated solutions are often stored in the laboratory stockroom for use as needed. – Frequently we dilute these “stock” solutions before working with them. – Dilution of solution is the procedure for …
Read More »50 MCQ on Chemical bonding
MCQ on Chemical bonding – In this subject, you will find 50 questions and answers MCQ on Chemical bonding 1. The valency of an element is ___________ (a) the combining capacity of one atom of it (b) the number of bonds formed by its one atom( c) the number …
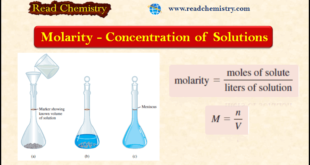
Read More »Molarity: definition, formula, solved Problems
– In this subject, we will discuss the Molarity: definition, formula, solved Problems – To study solution stoichiometry, we must know how much of the reactants are present in a solution and also how to control the amounts of reactants used to bring about a reaction in aqueous solution. The …
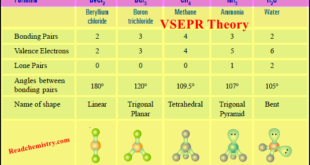
Read More »VSEPR Theory: Postulates, Predicting Shapes of Molecules
– In this subject, we will discuss the VSEPR Theory: definition, Postulates, limitations, and Predicting Shapes of Molecules. VSEPR Theory – The Lewis structure of a molecule tells us the number of pairs of electrons in the valence shell of the central atom. – These electron pairs are subject to …
Read More »Redox Reactions: Types, Examples, Applications, Balancing
– In this subject, we will discuss the Redox Reactions: Types, Examples, Applications, Balancing. Types of Redox Reactions – Among the most common oxidation-reduction reactions are: Combination Reactions Decomposition Reactions Combustion Reactions Displacement Reactions Disproportionation Reaction (1) Combination Reactions – A combination reaction is a reaction in which two or …
Read More » Read Chemistry
Read Chemistry