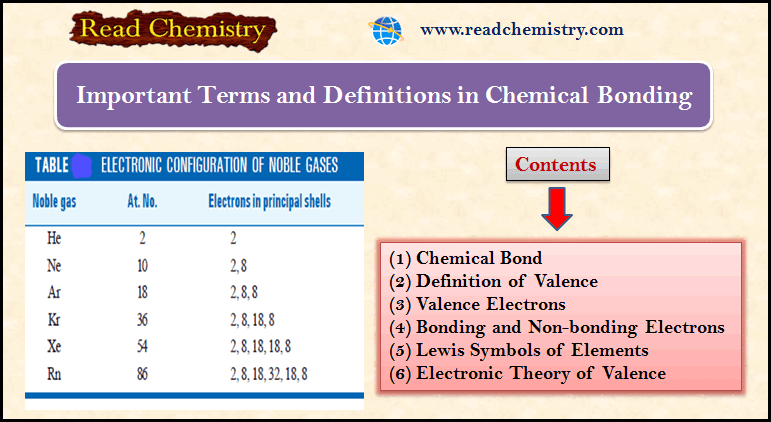
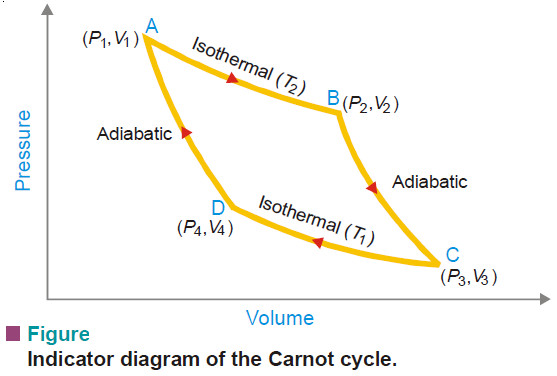
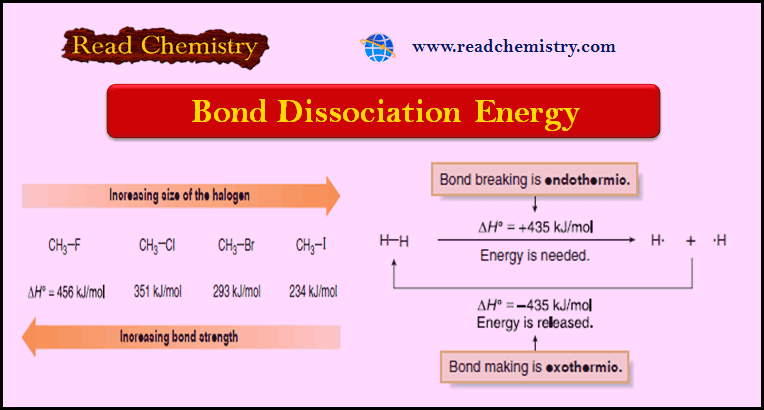
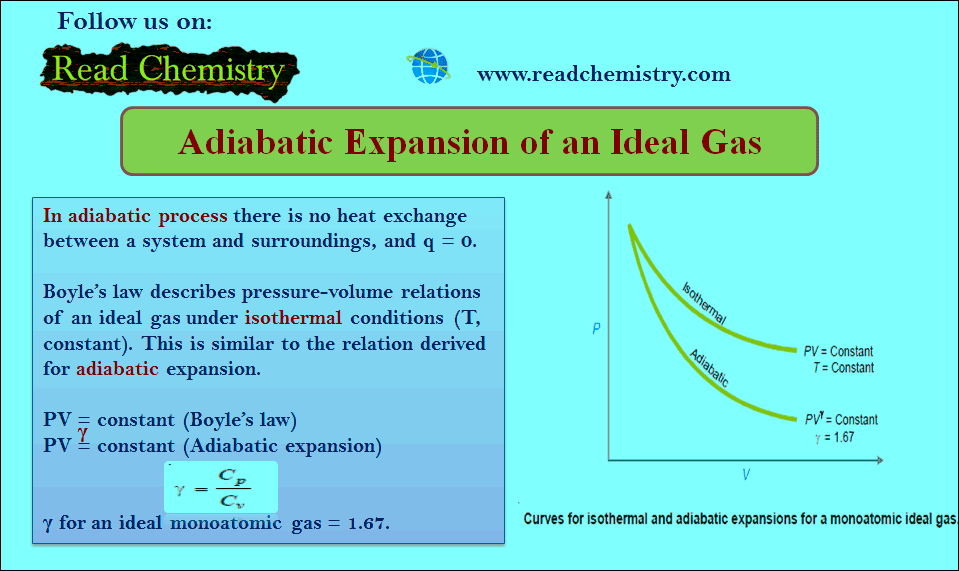
Popular Posts
-
Analytical Chemistry
The Laboratory Notebook
The Laboratory Notebook – A laboratory notebook is needed to record measurements and observations concerning an analysis. – The book…
Read More » -
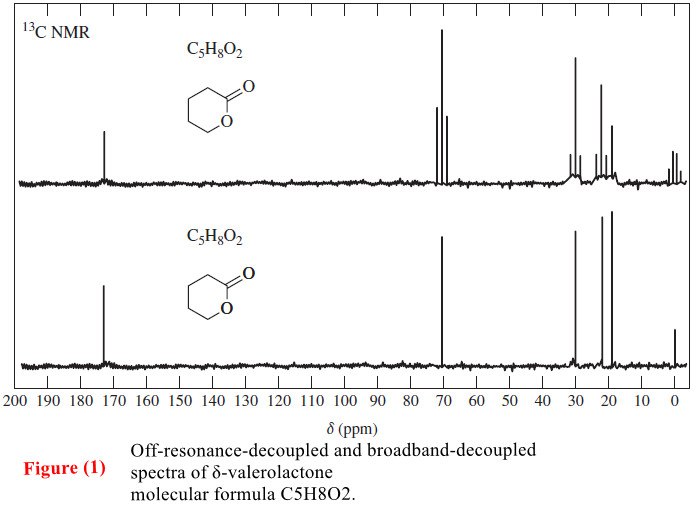
Organic Chemistry
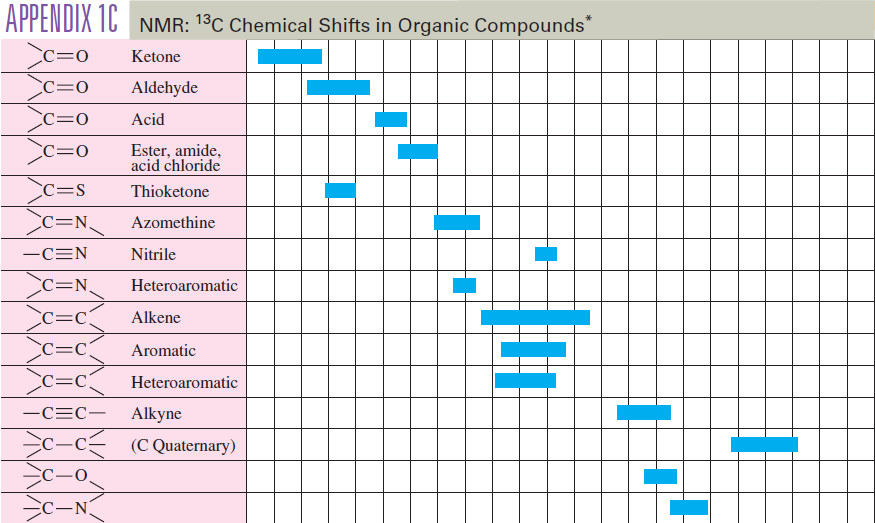
Carbon-13 NMR Spectroscopy
– In this topic, we will discuss The Carbon-13 NMR Spectroscopy. Carbon-13 NMR Spectroscopy – Where does a carbonyl group…
Read More » -
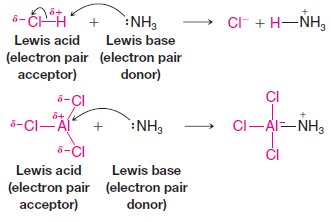
Organic Chemistry
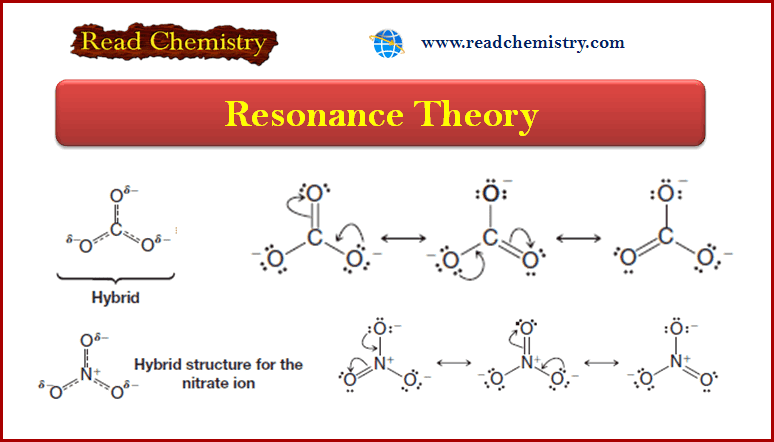
Resonance Theory
Introduction to Resonance (Resonance in carbonate ion (CO32-) ** Often more than one equivalent Lewis structure can be…
Read More » -
Physical Chemistry
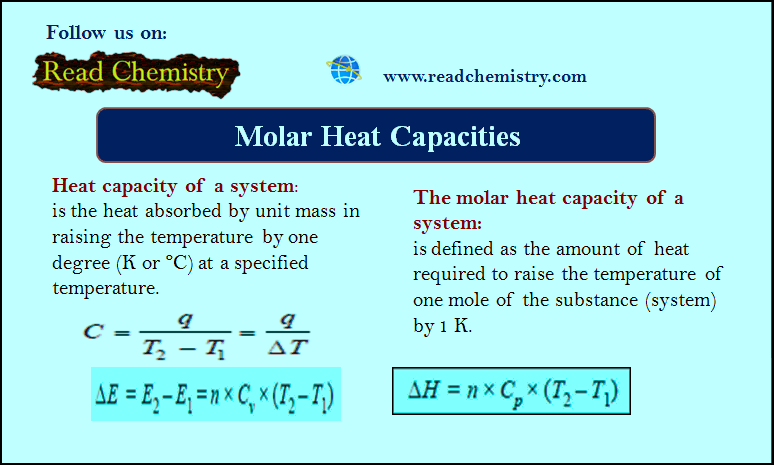
Heat Capacity – Molar Heat Capacity
Molar Heat Capacity – By heat capacity of a system, we mean the capacity to absorb heat and store energy.…
Read More » -
Physical Chemistry
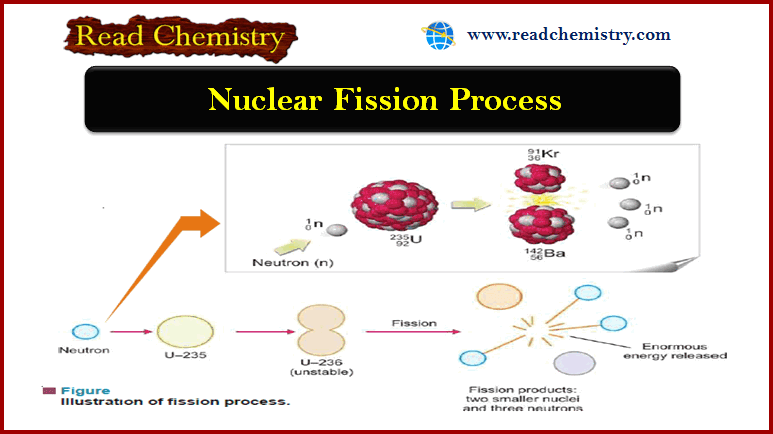
Nuclear Fission: Definition, Properties, Examples, Applications
– In this subject, we will discuss the Nuclear Fission (Definition, Properties, Examples, Applications) Nuclear Fission Process – In…
Read More » -
Free book
Physical Chemistry book , 3rd edition by Robert G. Mortimer
– In this subject, we will discuss free download of Physical Chemistry book, 3rd edition by Robert G. Mortimer The…
Read More »
-
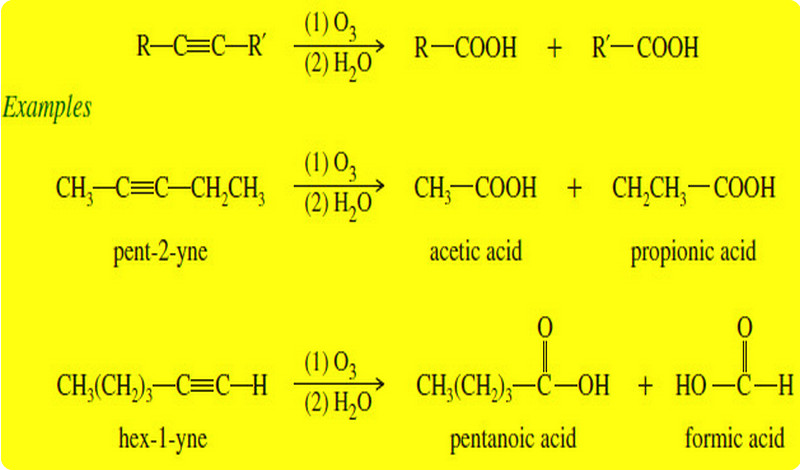
Organic Chemistry
Oxidation of Alkynes
Before we discuss Oxidation of Alkynes we will talk about triple bond of Alkynes What…
Read More » -
-
-
-
-
-
-
-
-
-
-
Physical Chemistry
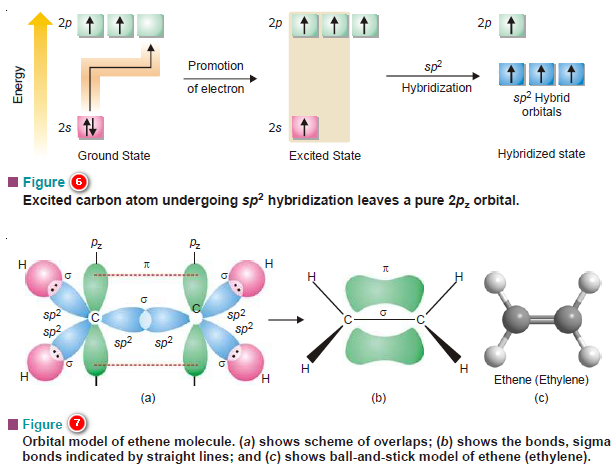
Hybridization and Shapes of Molecules
Hybridization and Shapes of Molecules – In the previous subject, we talked about the concept…
Read More » -
-
-
-
-
-
-
-
-
-
-
General Chemistry
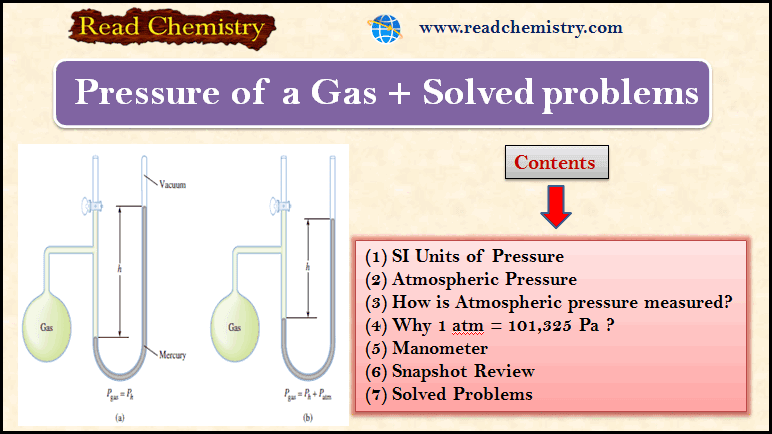
Gas Pressure: Definition, Formula and Solved problems
– In this subject, we will discuss Gas Pressure: Definition, Formula, and Solved problems Gas…
Read More » -
-
-
-
-
-
-
-
-
-
-
Analytical Chemistry
Safety in the laboratory
– In this subject, we will discuss the Safety in the laboratory Safety in The…
Read More » -
-
-
-
-
-
-
-
-
-
-
Online MCQ
First law of thermodynamics – MCQ online test
Online MCQ test on First law of thermodynamics – In this topic we offer you,…
Read More » -
-
-
-
-
-
-
-
Free book
Physical Chemistry book , 3rd edition by Robert G. Mortimer
– In this subject, we will discuss free download of Physical Chemistry book, 3rd edition…
Read More » -
-
-
-
-