Popular Posts
-
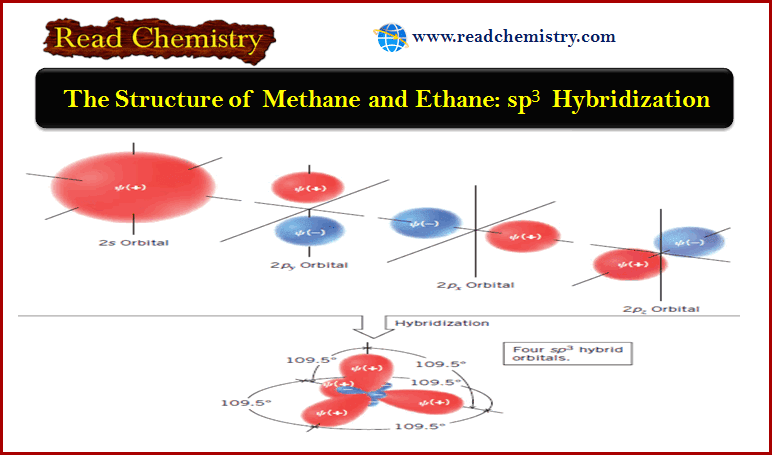
Organic Chemistry
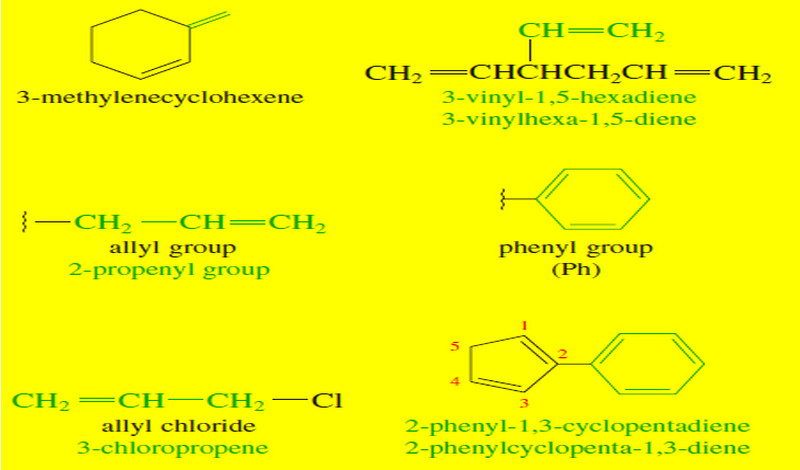
Nomenclature of Alkenes
Nomenclature of Alkenes – Simple alkenes are named much like alkanes, using the root name of the longest chain containing…
Read More » -
Online MCQ
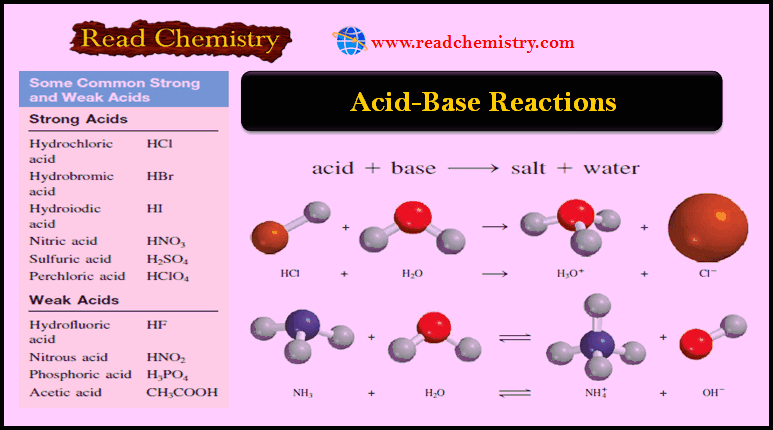
Acids and Bases – Online MCQ test
Online MCQ test on Acids and Bases – In this topic we offer you, online MCQ test in the fundmental…
Read More » -
General Chemistry
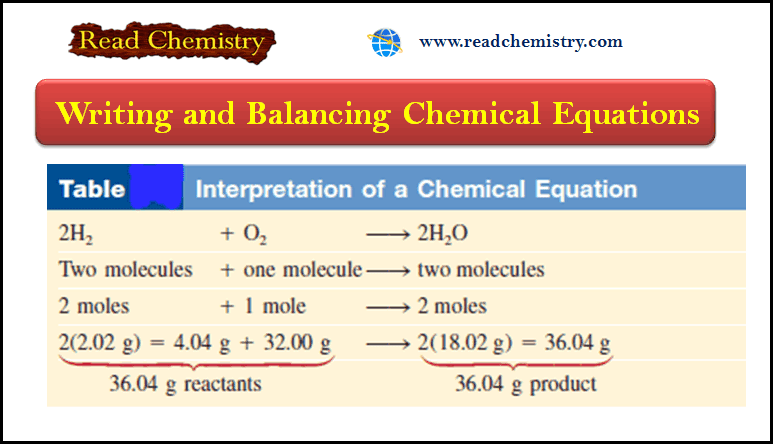
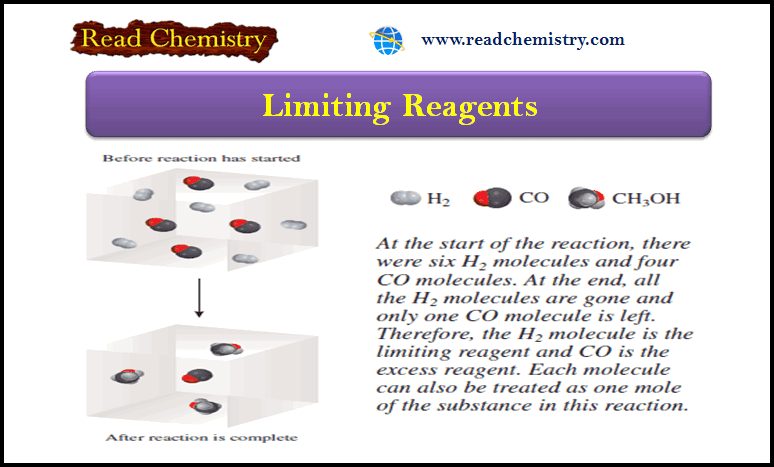
Chemical Equations – Writing and Balancing Chemical Equations
– In this subject, we will discuss Writing and Balancing Chemical Equations. Chemical Reactions and Chemical Equations – A chemical…
Read More » -
General Chemistry
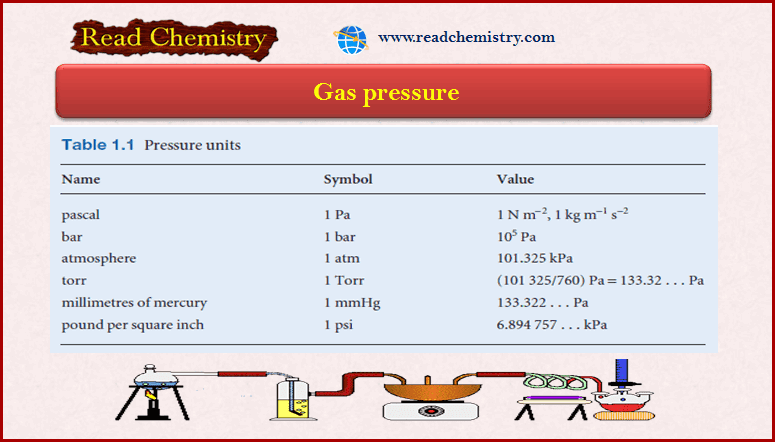
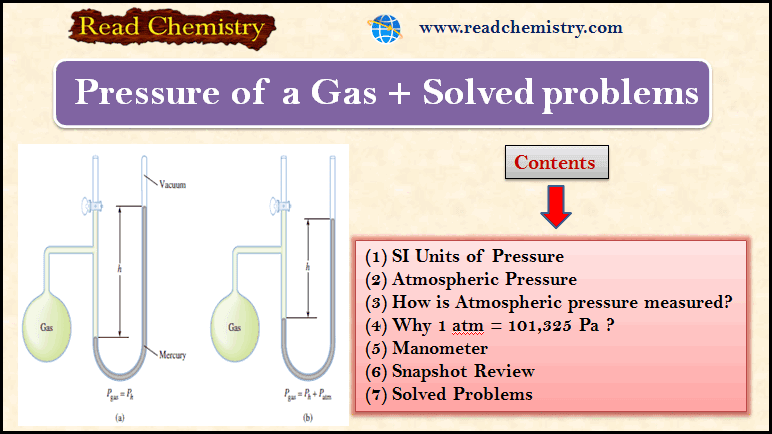
Gas Pressure: Definition, Units, Measurement
– In this subject, we will discuss Gas Pressure: Definition, Units, Measurement Key points of the Gas Pressure lesson…
Read More » -
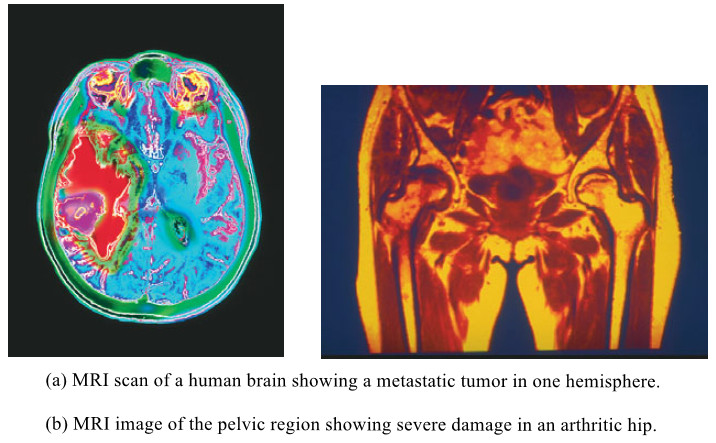
Biochemistry
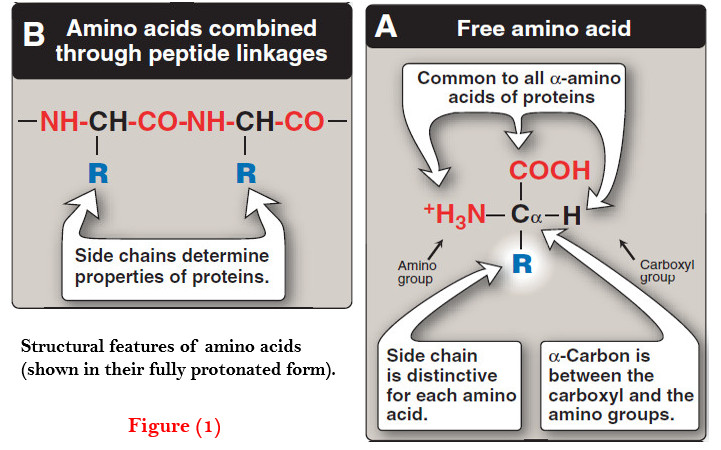
Amino acids – Structure of Amino acids
Structure of Amino acids – Although more than 300 different amino acids have been described in nature, only 20 are…
Read More » -
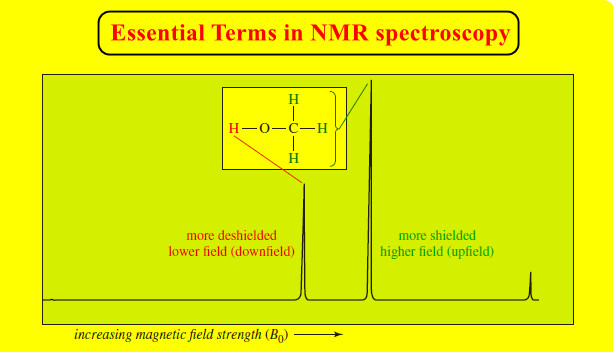
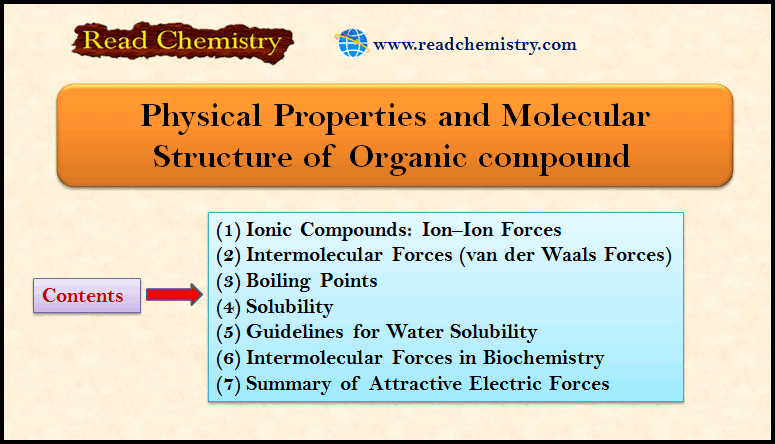
Physical Chemistry
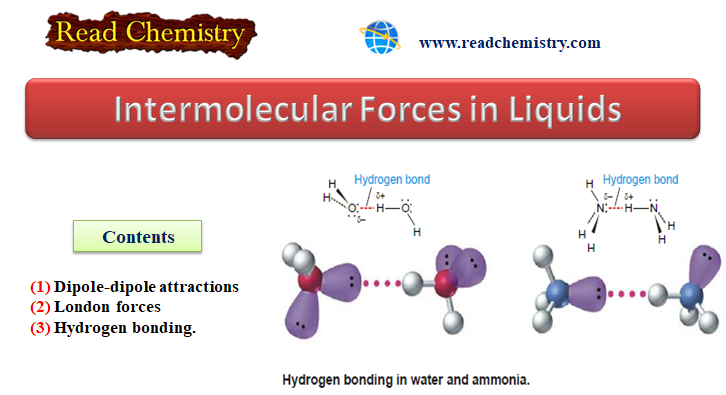
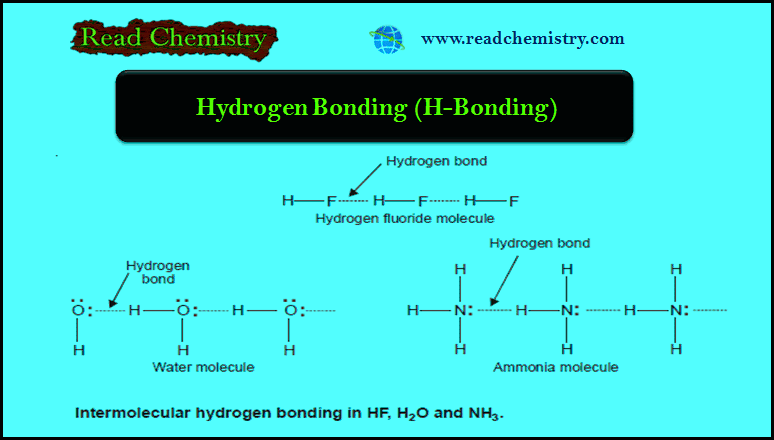
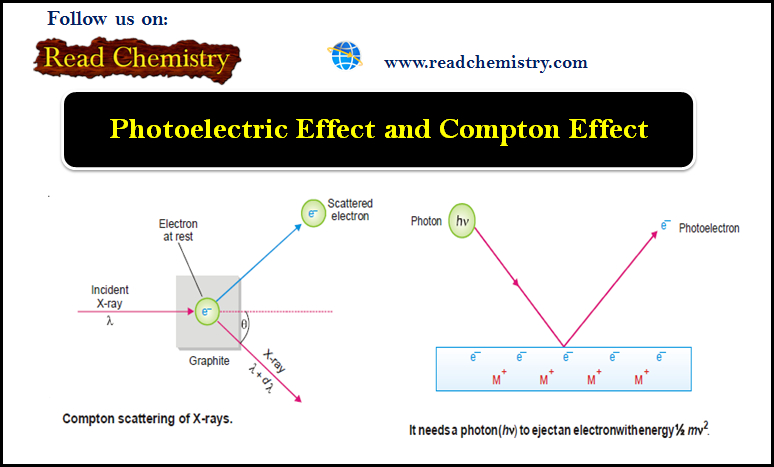
Intermolecular Forces in Liquids
Intermolecular Forces in Liquids – Intermolecular forces in liquids are collectively called van der Waals forces. – These forces are…
Read More »
-
Organic Chemistry
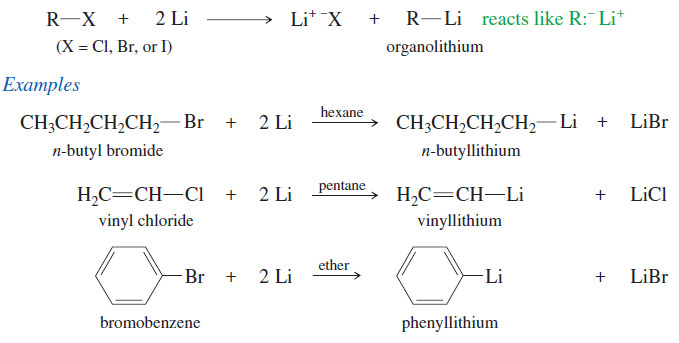
Organometallic Reagents for Alcohol Synthesis
Organometallic Reagents for Alcohol Synthesis – Organometallic compounds contain covalent bonds between carbon atoms and…
Read More » -
-
-
-
-
-
-
-
-
-
-
Physical Chemistry
Metallic Bonding: Definition, Properties, Examples, Explanation
– In this subject, we will discuss the Metallic Bonding: Definition, Properties, Examples, Explanation Metallic…
Read More » -
-
-
-
-
-
-
-
-
-
-
General Chemistry
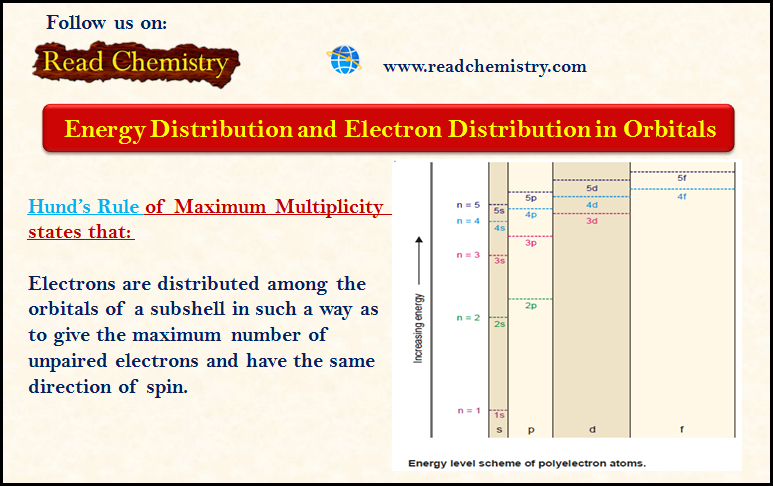
Distribution of Electrons in Orbitals
– In this subject, we will discuss the Distribution of Electrons in Orbitals according to…
Read More » -
-
-
-
-
-
-
-
-
-
-
Analytical Chemistry
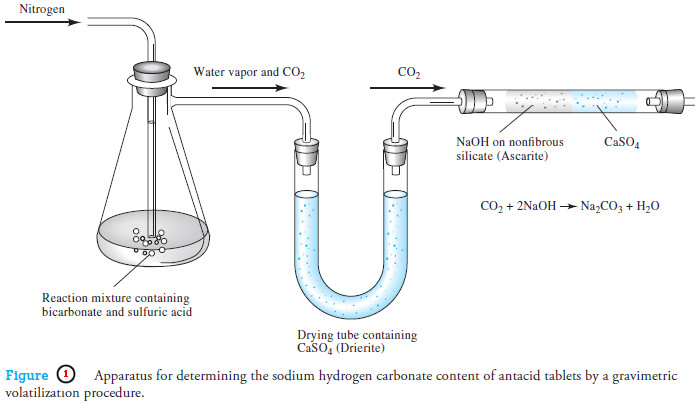
Applications of Neutralization Titrations
– In this topic, we will discuss The Applications of Neutralization Titrations. Typical Applications of…
Read More » -
-
-
-
-
-
-
-
-
-
-
Online MCQ
First law of thermodynamics – MCQ online test
Online MCQ test on First law of thermodynamics – In this topic we offer you,…
Read More » -
-
-
-
-
-
-
-
Free book
Physical Chemistry book , 3rd edition by Robert G. Mortimer
– In this subject, we will discuss free download of Physical Chemistry book, 3rd edition…
Read More » -
-
-
-
-