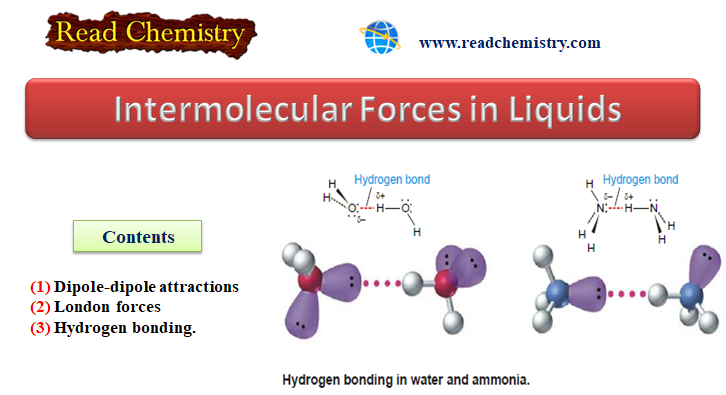
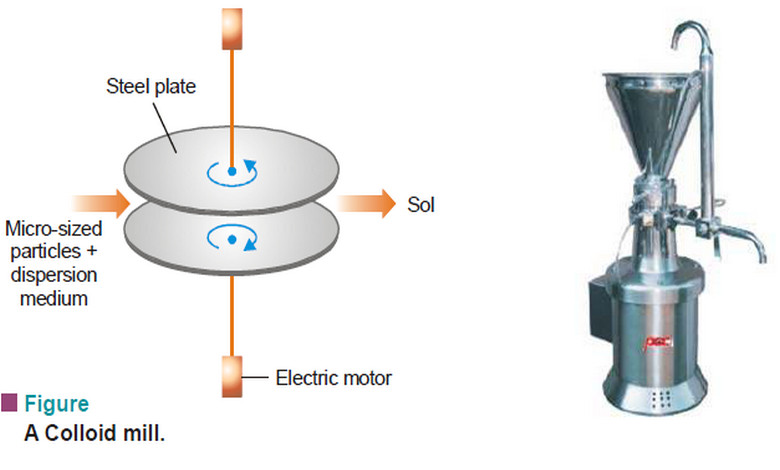
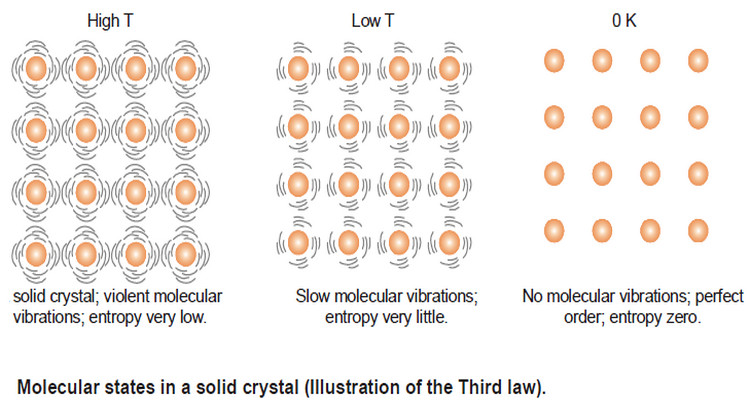
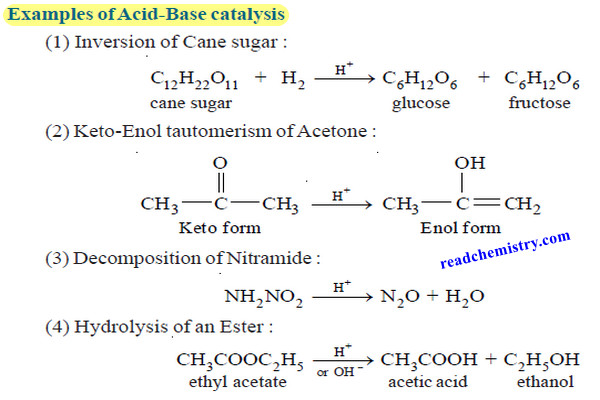
Popular Posts
-
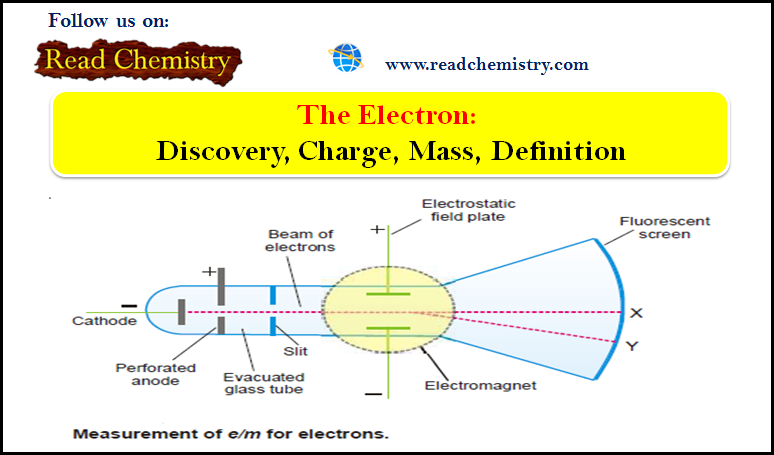
General Chemistry
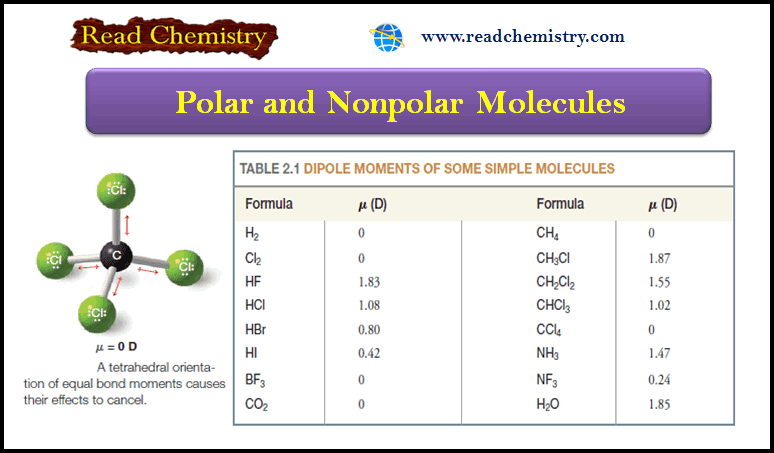
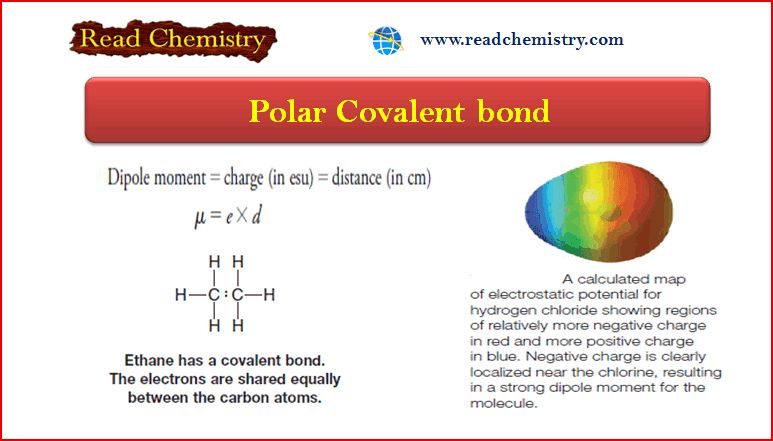
Polar and Nonpolar Molecules
– In this subject, we will discuss the Polar and Nonpolar Molecules. Dipole moment – The dipole moment is a…
Read More » -
Organic Chemistry
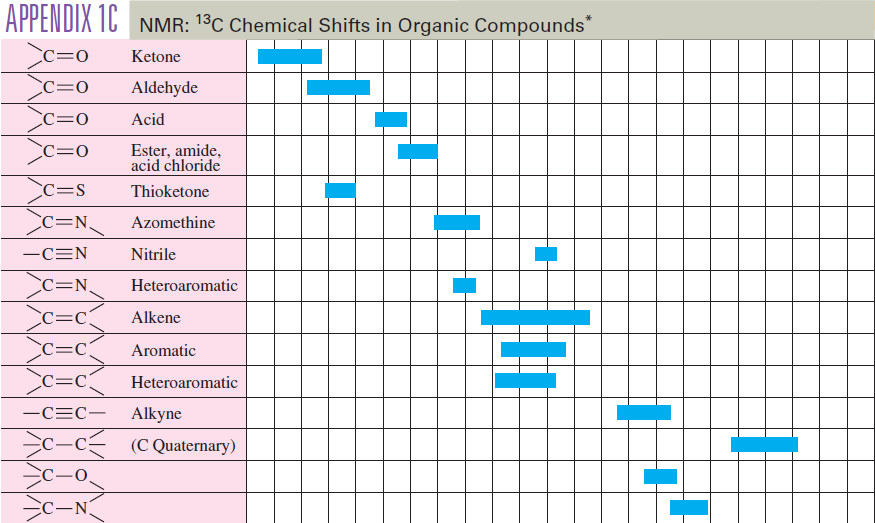
Carbon-13 NMR Spectroscopy
– In this topic, we will discuss The Carbon-13 NMR Spectroscopy. Carbon-13 NMR Spectroscopy – Where does a carbonyl group…
Read More » -
Online MCQ
Chemical Bonding – Lewis Theory – Online MCQ test
Online MCQ test on Chemical Bonding – Lewis Theory – In this topic we offer you, online MCQ test in…
Read More » -
Organic Chemistry
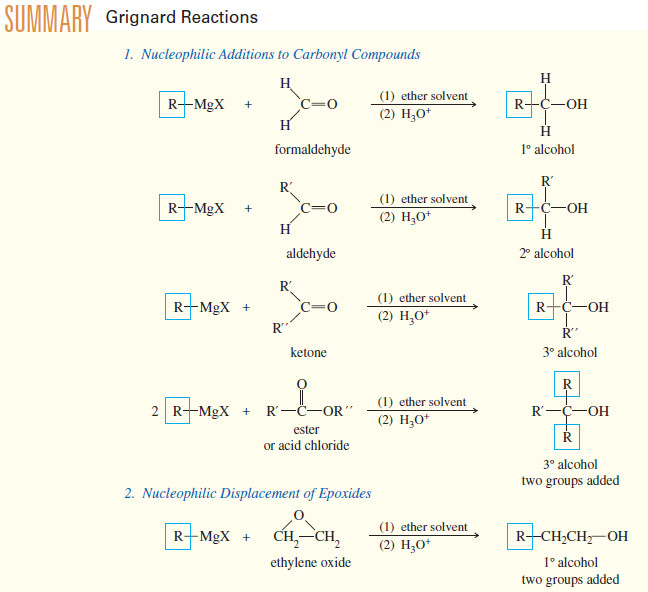
Addition of Grignard Reagents to Carbonyl Compounds
Addition of Organometallic Reagents to Carbonyl Compounds – Because they resemble carbanions, Grignard reagents and organolithium reagents are strong nucleophiles…
Read More » -
General Chemistry
MCQ on Chemical Bonding – Orbital Theory
MCQ on Chemical Bonding – Orbital Theory – In this subject, you will find 46 questions and answers MCQ on…
Read More » -
General Chemistry
How to Predict the Outcome of acid-base reaction
– In this subject, we will discuss How to Predict the Outcome of acid-base reaction. How To Predict the Outcome…
Read More »
-
Organic Chemistry
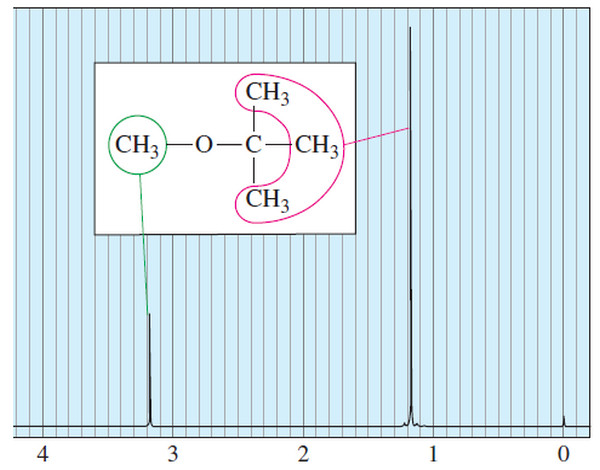
Number of Signals in NMR Spectroscopy
The Number of Signals – In general, the number of NMR signals corresponds to the…
Read More » -
-
-
-
-
-
-
-
-
-
-
Physical Chemistry
Gases – General Characteristics of gases
States of the matter – All matter exists in three states: gases, liquids and solids.…
Read More » -
-
-
-
-
-
-
-
-
-
-
General Chemistry
Molecular Orbitals for Homonuclear Diatomic Molecules
Molecular Orbitals for Homonuclear Diatomic Molecules – In the previous subject, we talk about but…
Read More » -
-
-
-
-
-
-
-
-
-
-
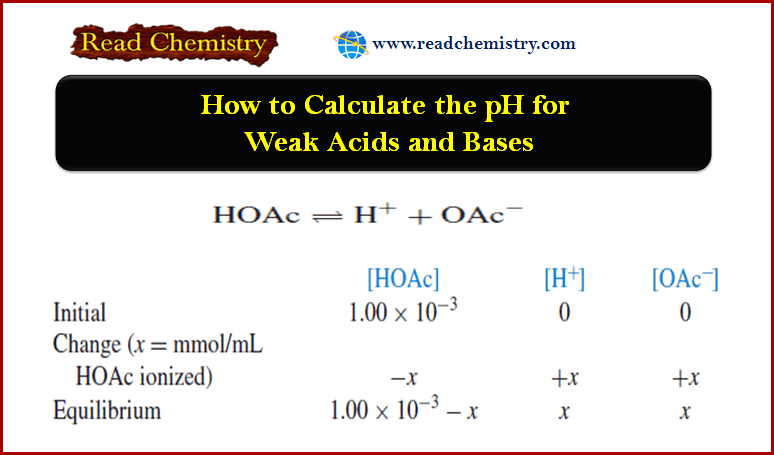
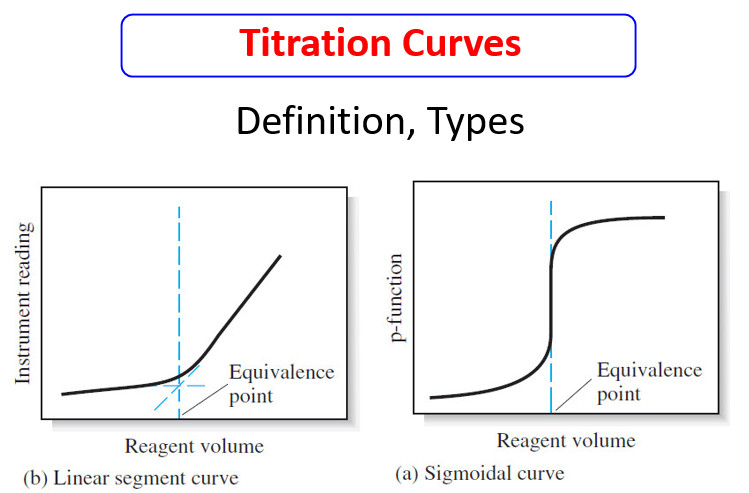
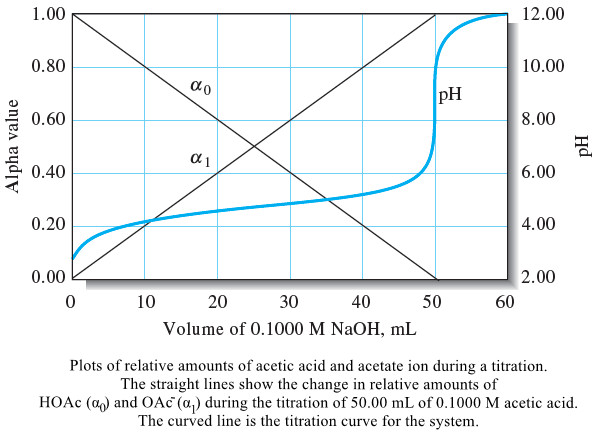
Analytical Chemistry
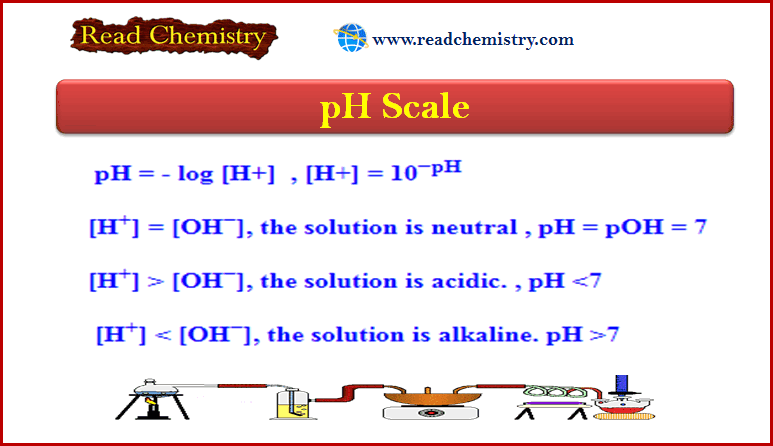
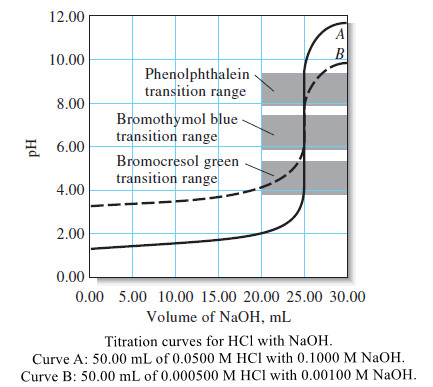
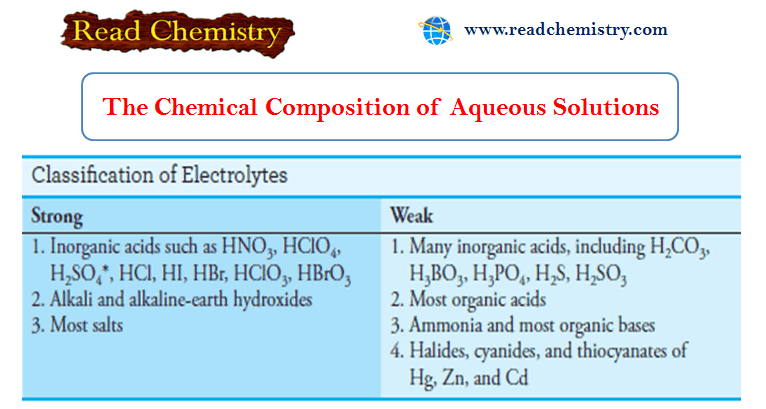
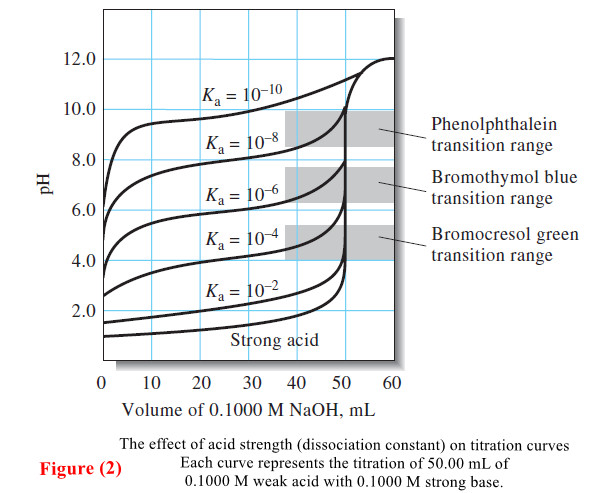
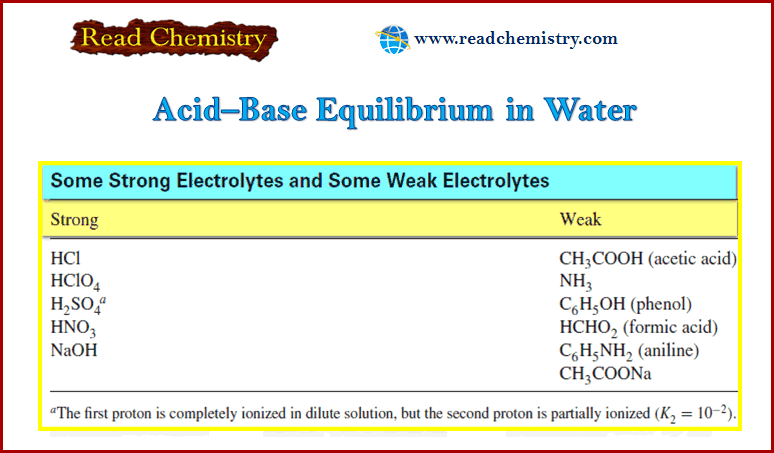
Calculating the pH of Weak Acid and Base Solutions
– In this subject, we will discuss Calculating the pH of Weak Acid and Base…
Read More » -
-
-
-
-
-
-
-
-
-
-
Online MCQ
First law of thermodynamics – MCQ online test
Online MCQ test on First law of thermodynamics – In this topic we offer you,…
Read More » -
-
-
-
-
-
-
-
Free book
Physical Chemistry book , 3rd edition by Robert G. Mortimer
– In this subject, we will discuss free download of Physical Chemistry book, 3rd edition…
Read More » -
-
-
-
-