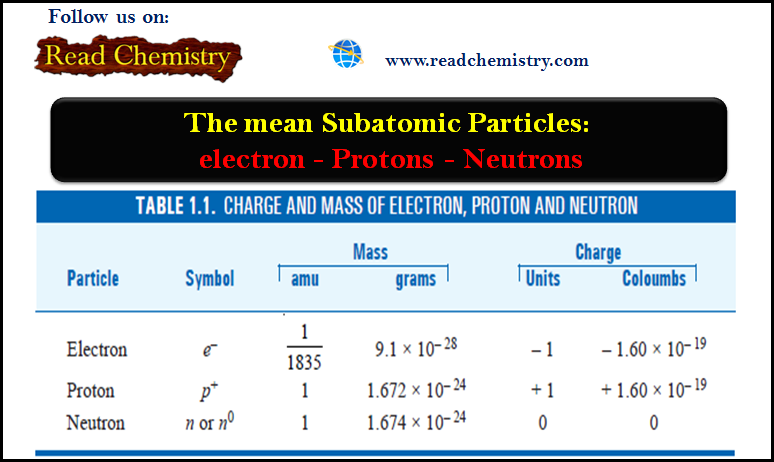
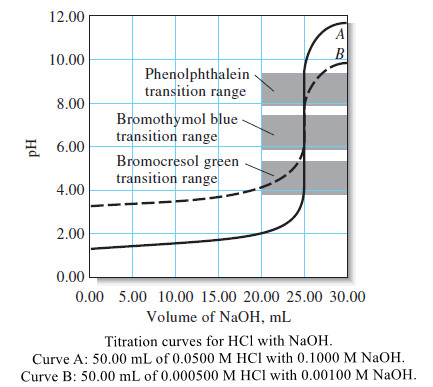
Popular Posts
-
General Chemistry
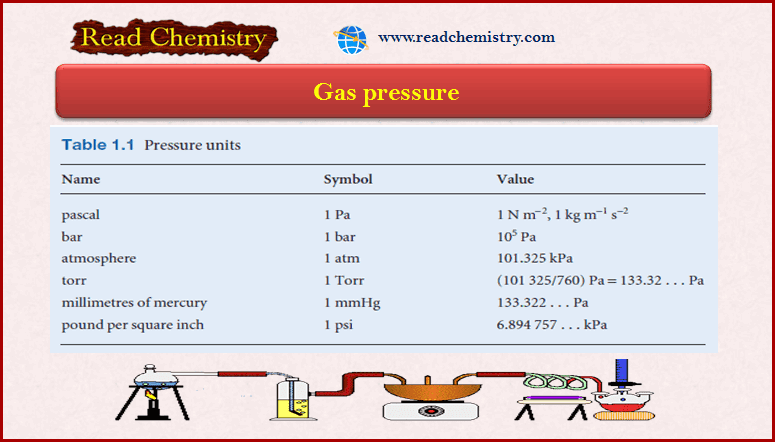
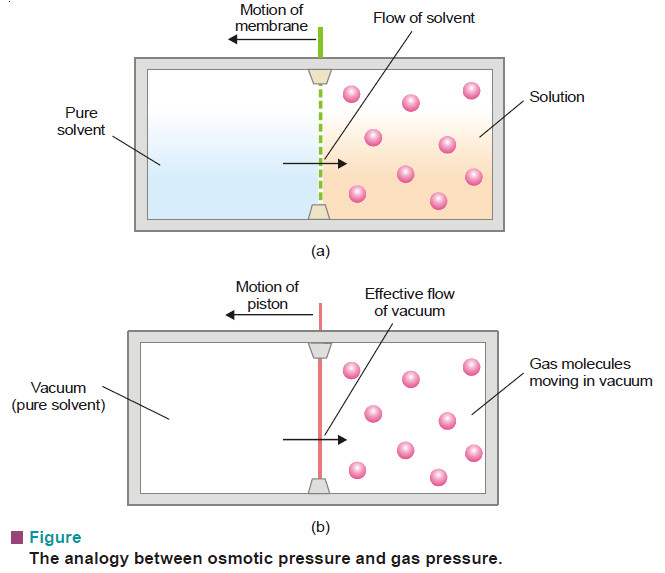
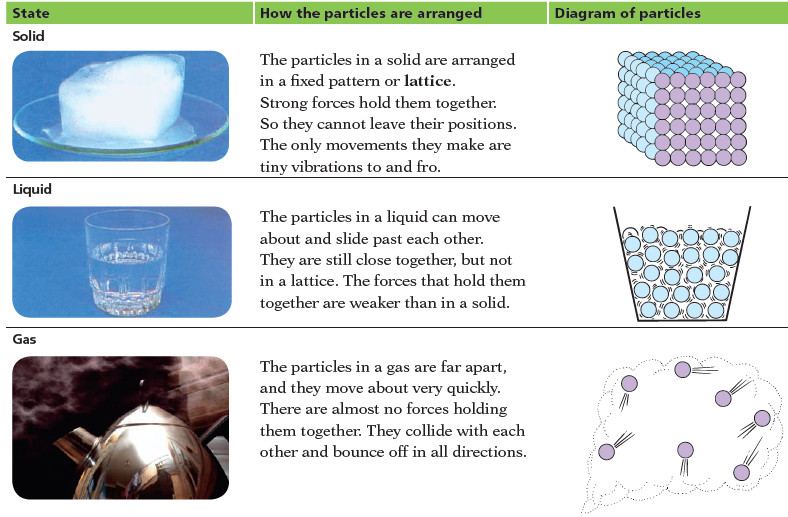
Gas Pressure: Definition, Units, Measurement
– In this subject, we will discuss Gas Pressure: Definition, Units, Measurement Key points of the Gas Pressure lesson…
Read More » -
Physical Chemistry
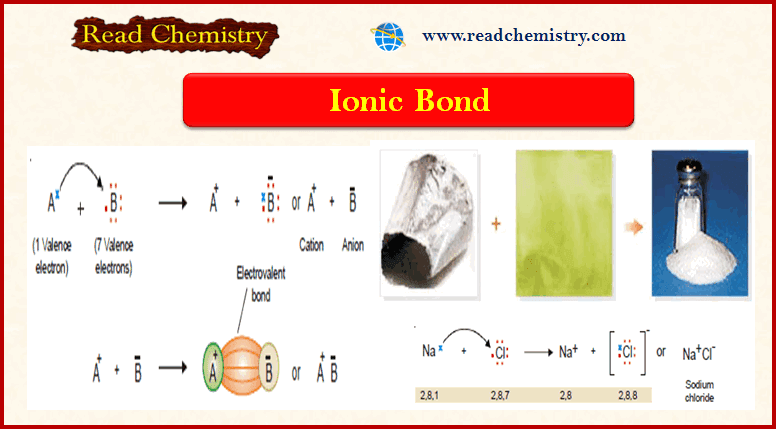
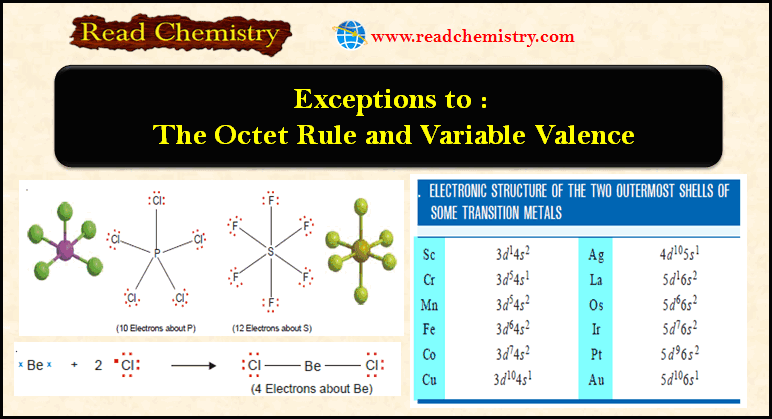
Ionic Bond: Definition, Examples, Types, Properties
– In this subject, we will discuss the Ionic Bond (Definition, Examples, Types, Properties) Ionic Bond – This type…
Read More » -
General Chemistry
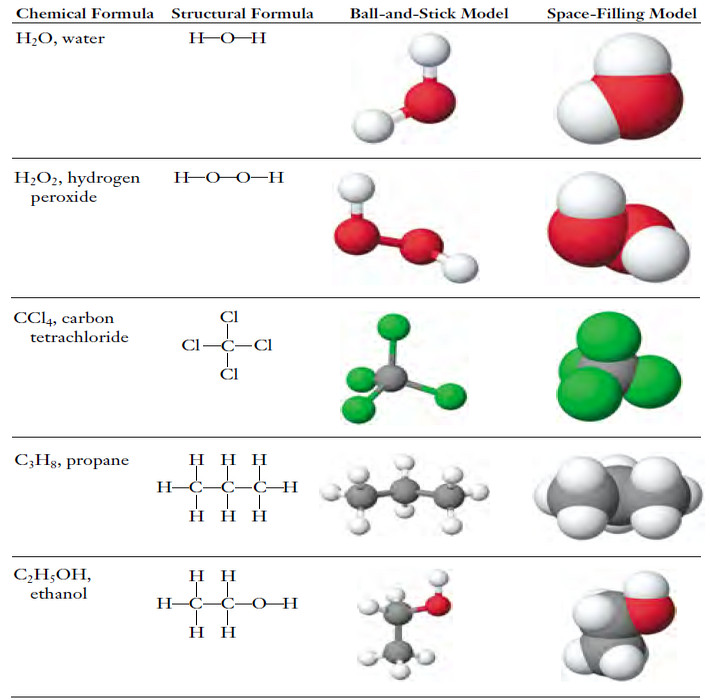
Chemical Formula – Structural Formula
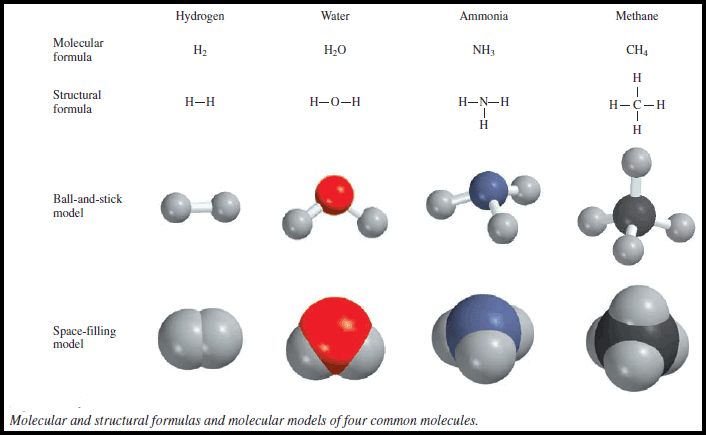
Chemical Formula – The chemical formula for a substance shows its chemical composition. – This represents the elements present as…
Read More » -
Physical Chemistry
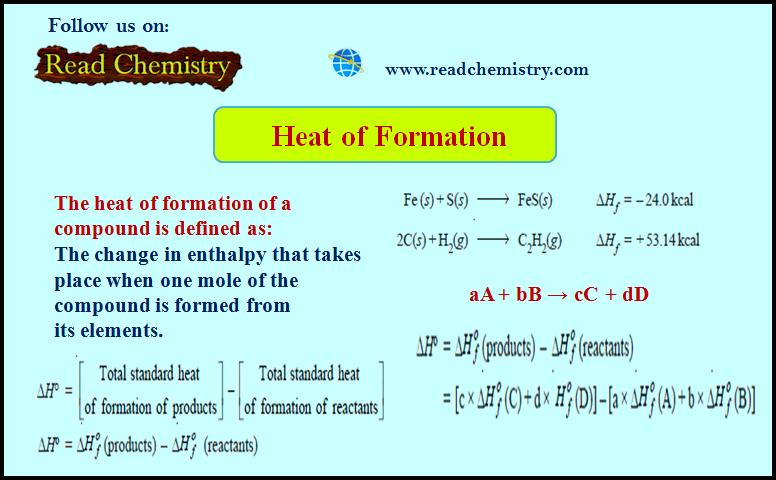
Heat of Formation (Definition, Applications, Solved Problems)
Heat of Formation – The heat of formation of a compound is defined as The change in enthalpy that takes…
Read More » -
General Chemistry
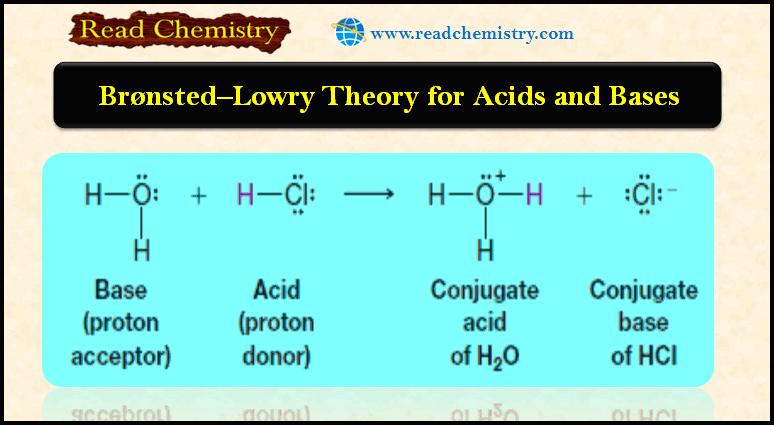
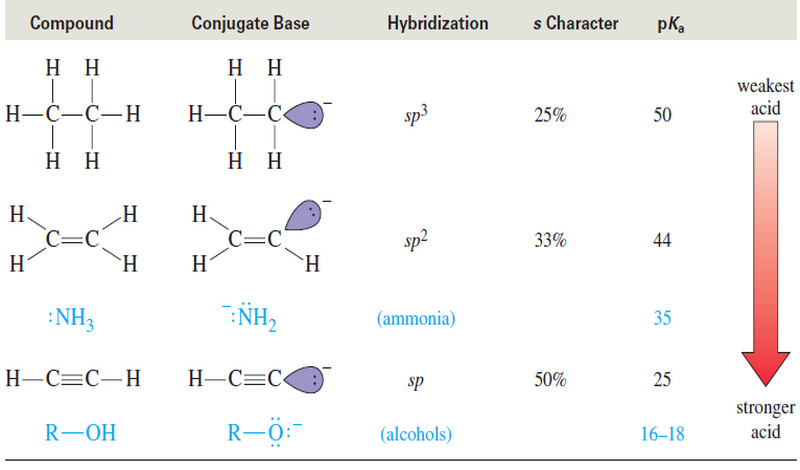
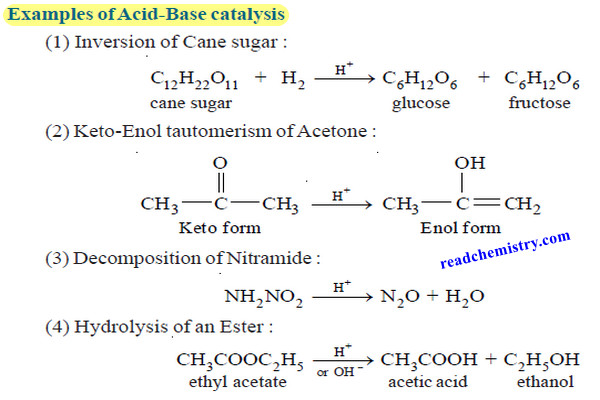
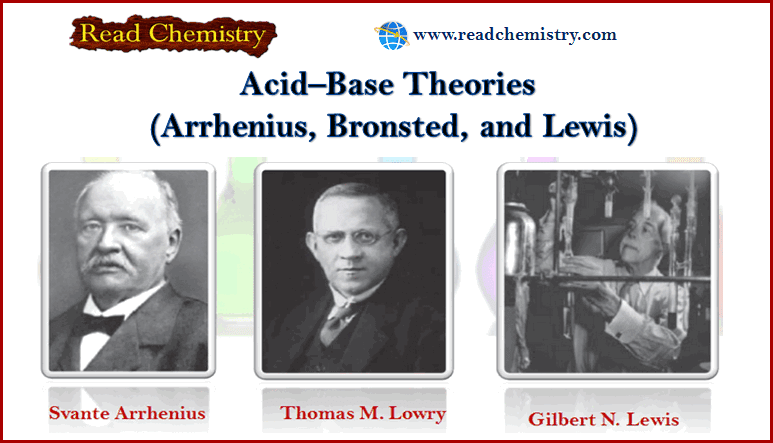
Bronsted-Lowry theory for acids and bases
– In this subject, we will discuss Bronsted-Lowry theory for acids and bases Acid-base reactions – We begin our study…
Read More » -
Physical Chemistry
Polymorphism – Allotropy
Polymorphism – The occurrence of the same substance in more than one crystalline forms is known as Polymorphism. – Polymorphism…
Read More »
-
Organic Chemistry
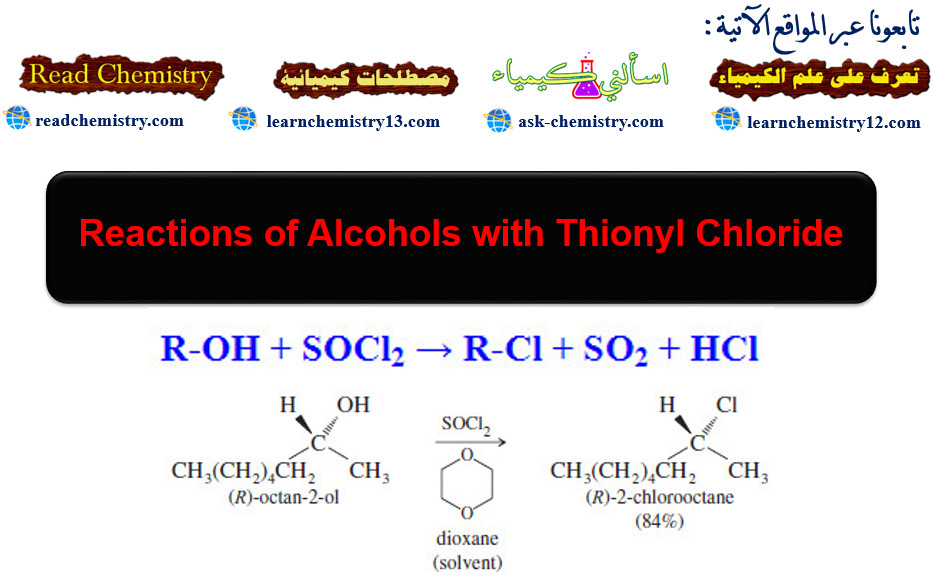
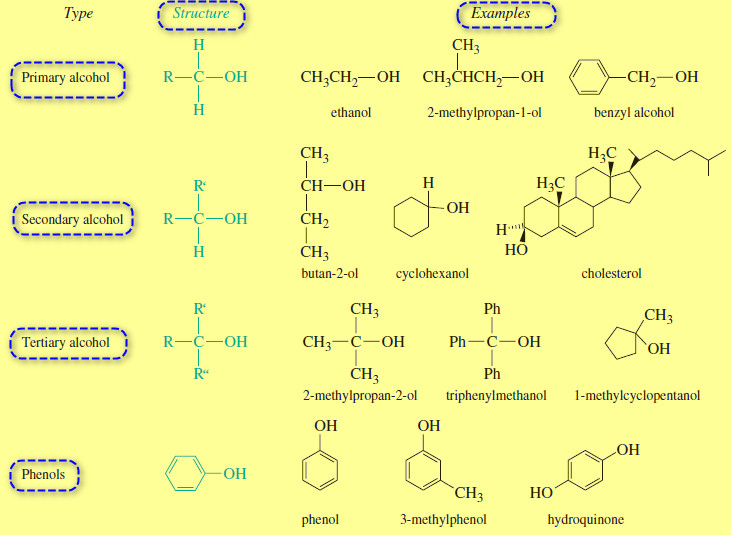
Reactions of Alcohols with Thionyl Chloride
Reactions of Alcohols with Thionyl Chloride – Reactions of Alcohols with Thionyl Chloride give alkyl…
Read More » -
-
-
-
-
-
-
-
-
-
-
Physical Chemistry
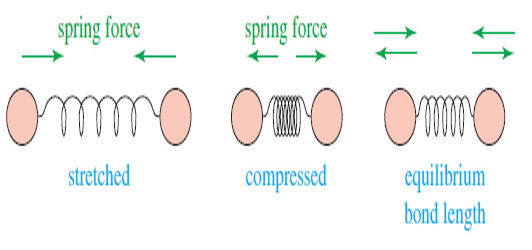
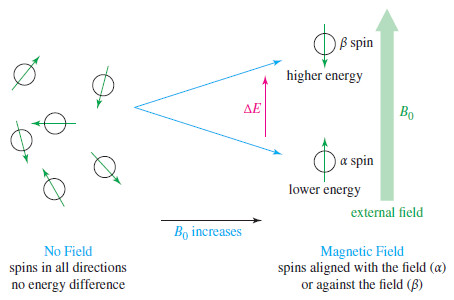
Molecular Vibrations : IR spectrum
– In this subject we talk about Molecular Vibrations as introduction to understand IR spectrum…
Read More » -
-
-
-
-
-
-
-
-
-
-
General Chemistry
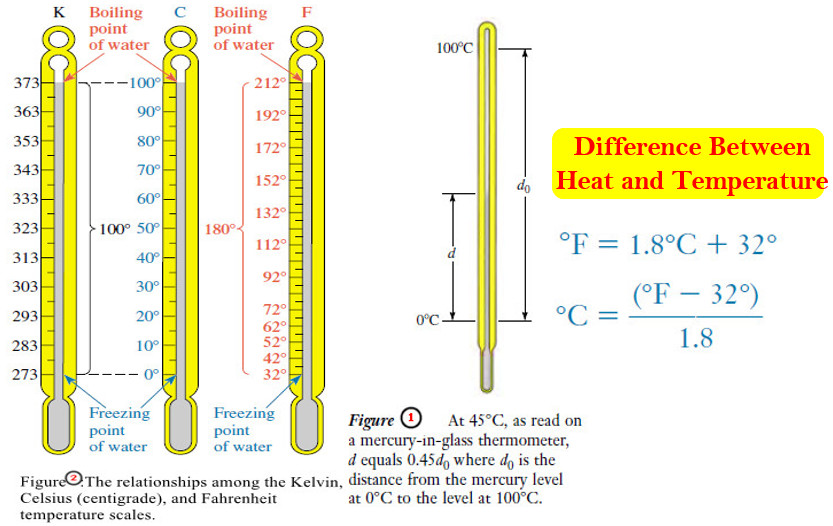
Heat and Temperature
Difference Between Heat and Temperature – Heat is one form of energy. – Many forms…
Read More » -
-
-
-
-
-
-
-
-
-
-
Analytical Chemistry
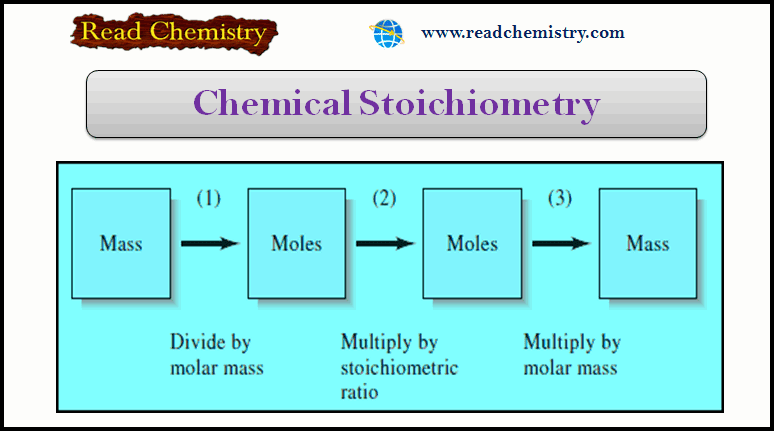
Chemical Stoichiometry: Definition, Formula, Examples
In this subject, we will discuss the Chemical Stoichiometry: Definition, Formula, Example Chemical Stoichiometry –…
Read More » -
-
-
-
-
-
-
-
-
-
-
Online MCQ
First law of thermodynamics – MCQ online test
Online MCQ test on First law of thermodynamics – In this topic we offer you,…
Read More » -
-
-
-
-
-
-
-
Free book
Physical Chemistry book , 3rd edition by Robert G. Mortimer
– In this subject, we will discuss free download of Physical Chemistry book, 3rd edition…
Read More » -
-
-
-
-