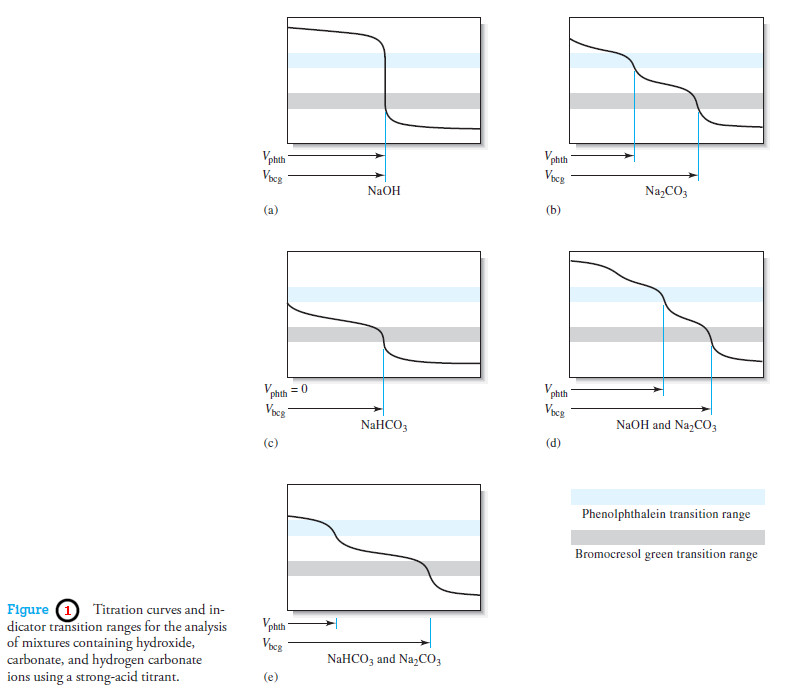
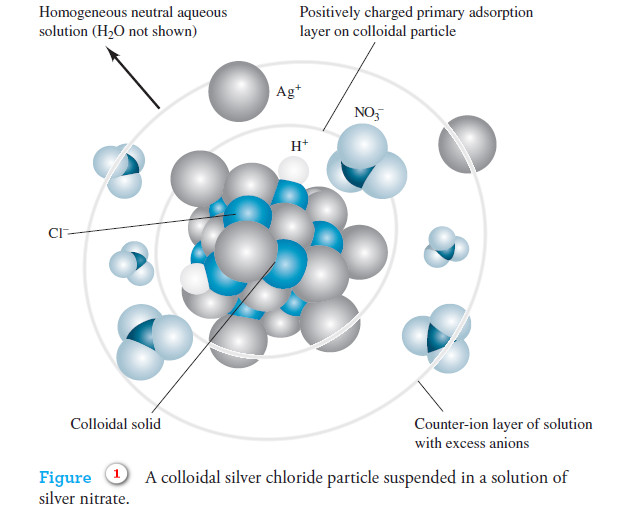
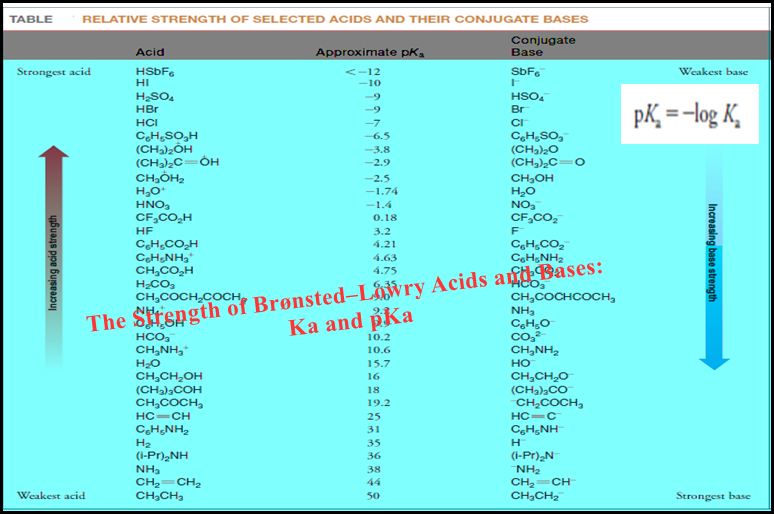
Popular Posts
-
Analytical Chemistry
Pipets : Overview, Uses, Function, Cleaning
Pipets – Pipets permit the transfer of accurately known volumes from one container to another. – Common types are shown…
Read More » -
Analytical Chemistry
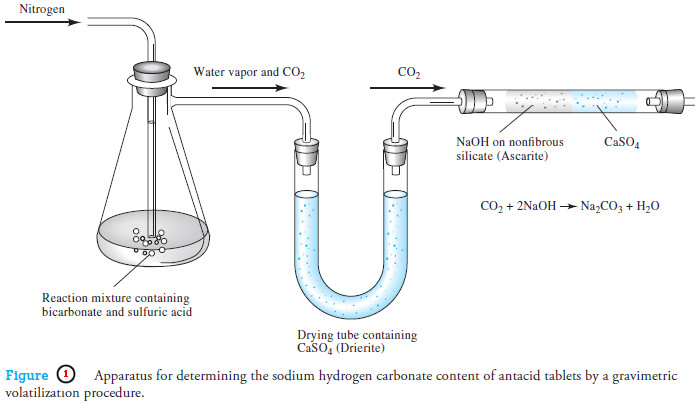
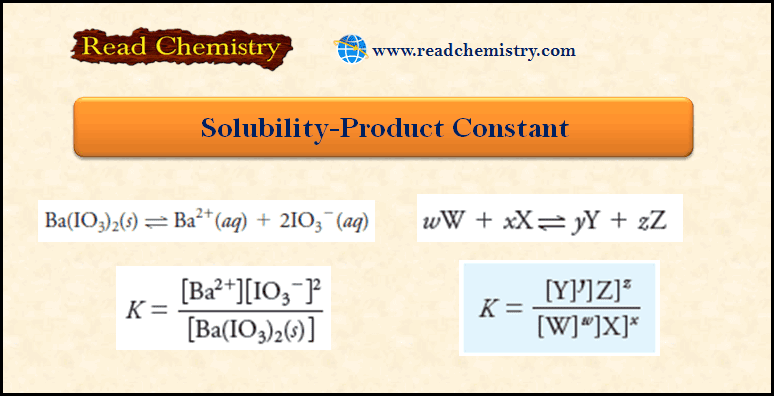
Applications of Gravimetric methods
Applications of Gravimetric methods – Gravimetric methods have been developed for most inorganic anions and cations, as well as for…
Read More » -
Organic Chemistry
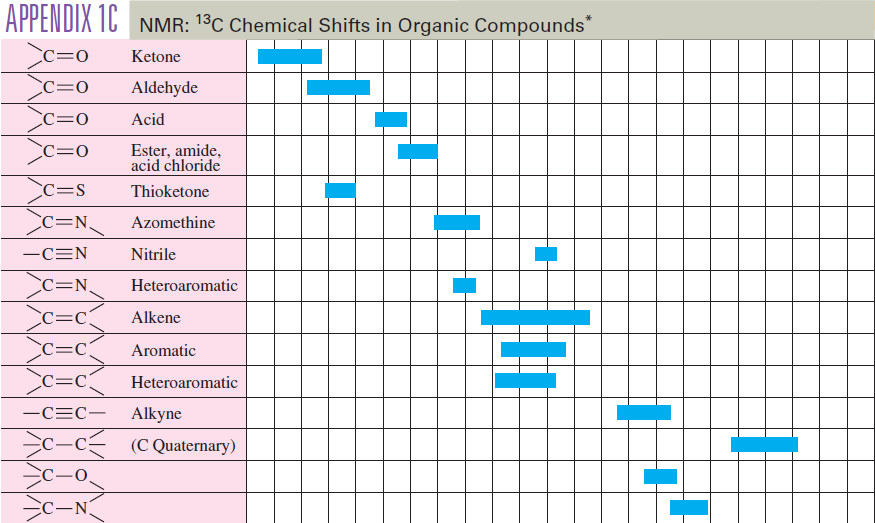
Carbon-13 NMR Spectroscopy
– In this topic, we will discuss The Carbon-13 NMR Spectroscopy. Carbon-13 NMR Spectroscopy – Where does a carbonyl group…
Read More » -
General Chemistry
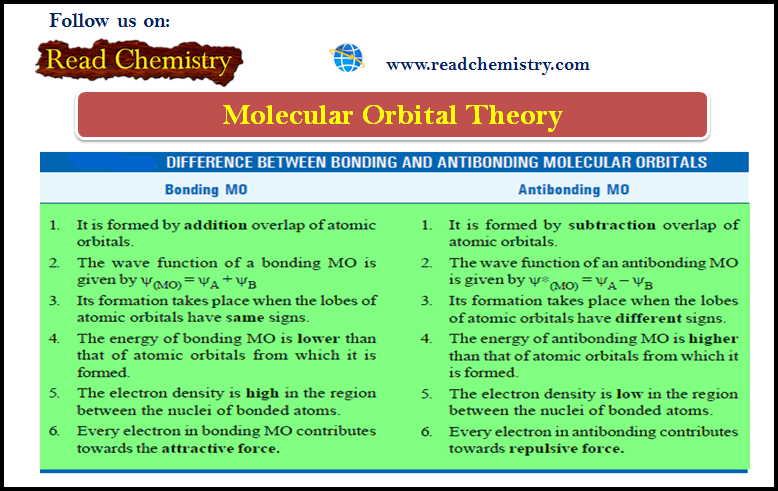
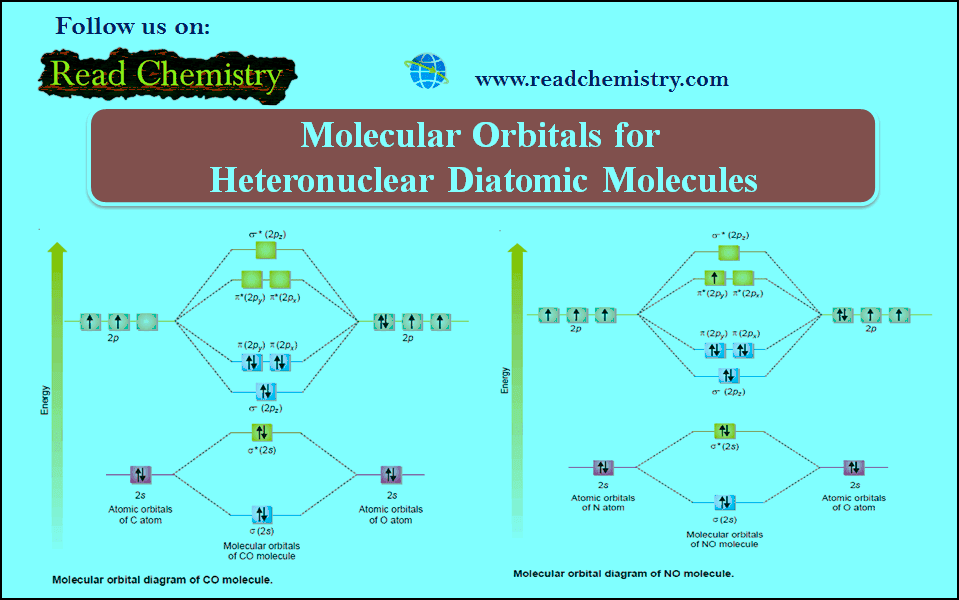
Molecular Orbital Theory
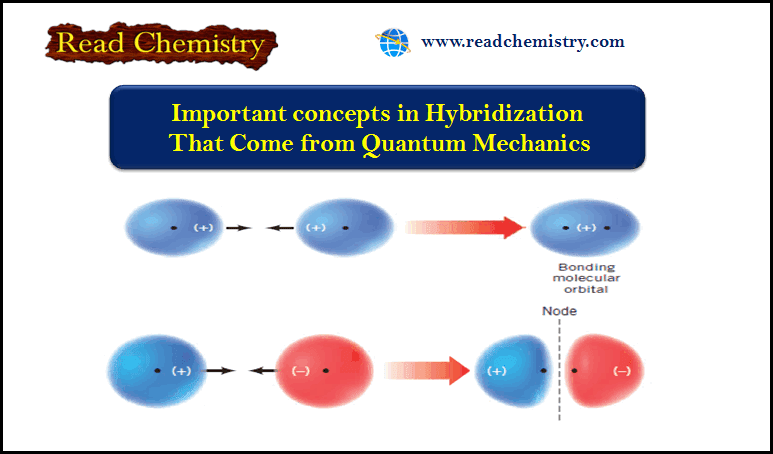
Molecular Orbital Theory – The molecular orbital theory proposed by Hund and Mulliken in 1932 explains the formation of a…
Read More » -
General Chemistry
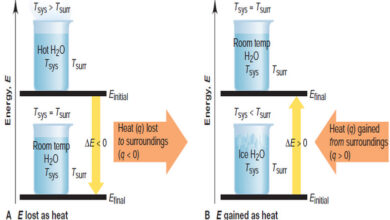
Forms of Energy and Their Interconversion
Forms of Energy and Their Interconversion – we discussed before the facts that all energy is either potential or kinetic…
Read More » -
Organic Chemistry
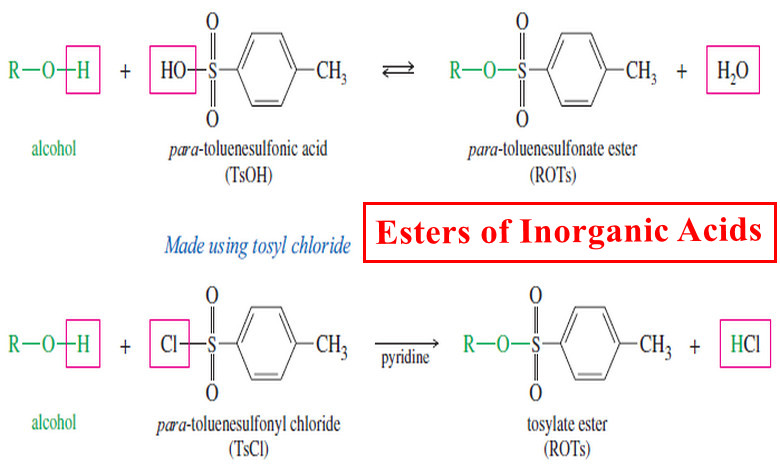
Inorganic Esters – Esters of Inorganic Acids
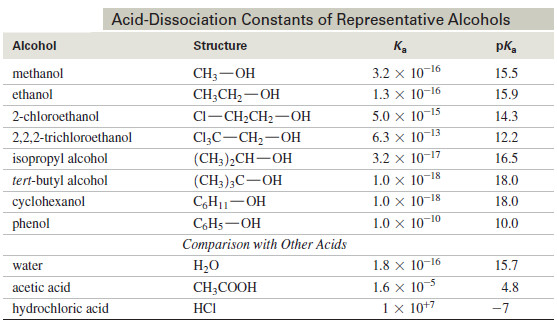
Esters of Inorganic Acids – In addition to forming esters with carboxylic acids, alcohols form inorganic esters with inorganic acids…
Read More »
-
Organic Chemistry
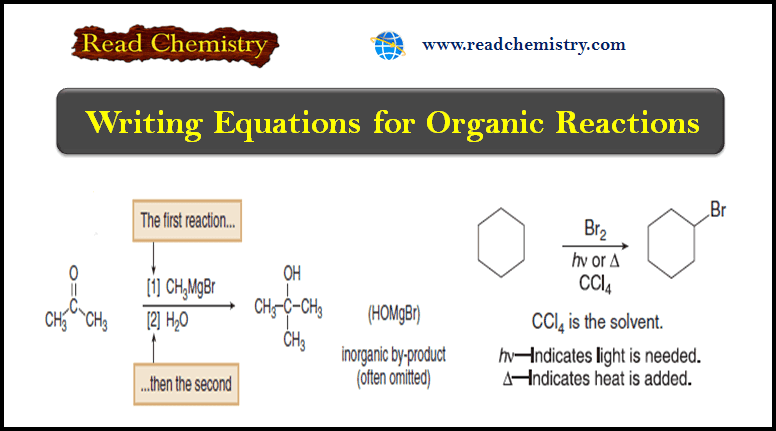
Writing Equations for Organic Reactions
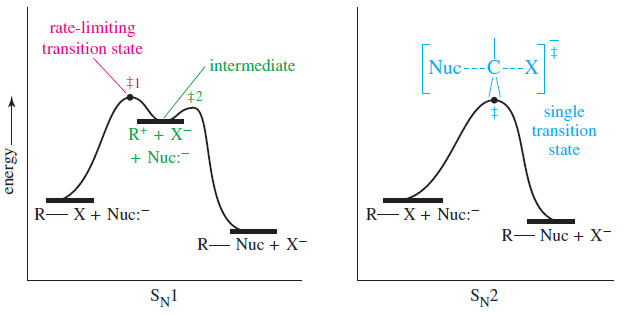
– In this subject, we will discuss Writing Equations for Organic Reactions. Writing Equations for…
Read More » -
-
-
-
-
-
-
-
-
-
-
Physical Chemistry
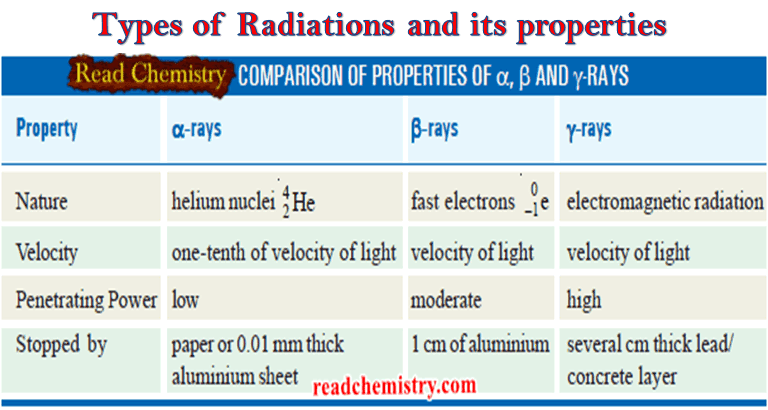
Types of Radiations and its properties
Nuclear reaction ** A nuclear reaction is different from a chemical reaction. **…
Read More » -
-
-
-
-
-
-
-
-
-
-
General Chemistry
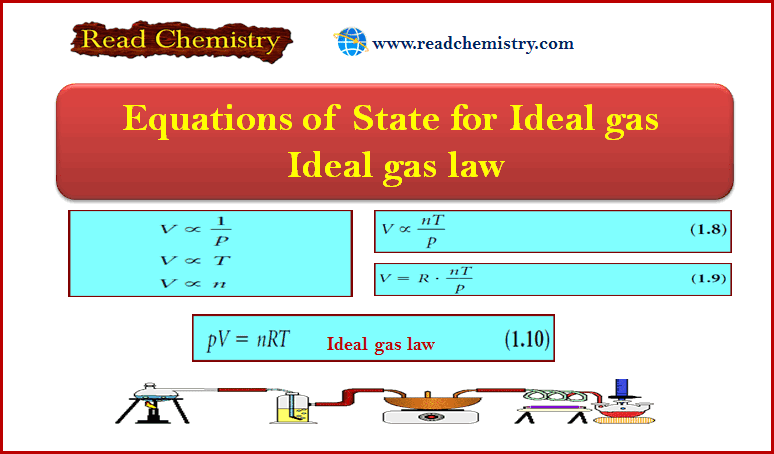
Equations of State for Ideal gas – Ideal gas law
Equations of State for Ideal gas ** Phenomenological thermodynamics is based on experiment, on…
Read More » -
-
-
-
-
-
-
-
-
-
-
Analytical Chemistry
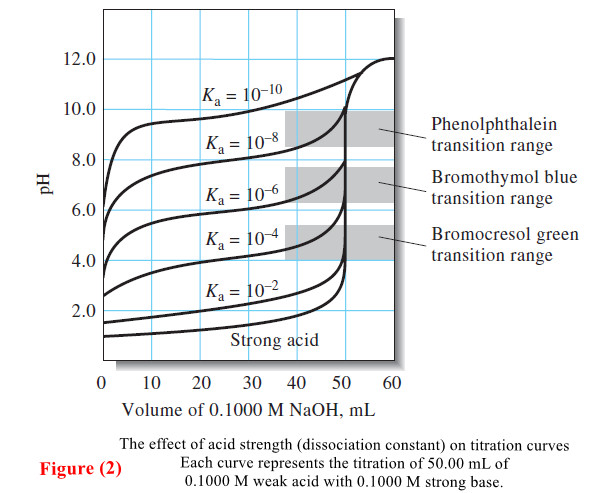
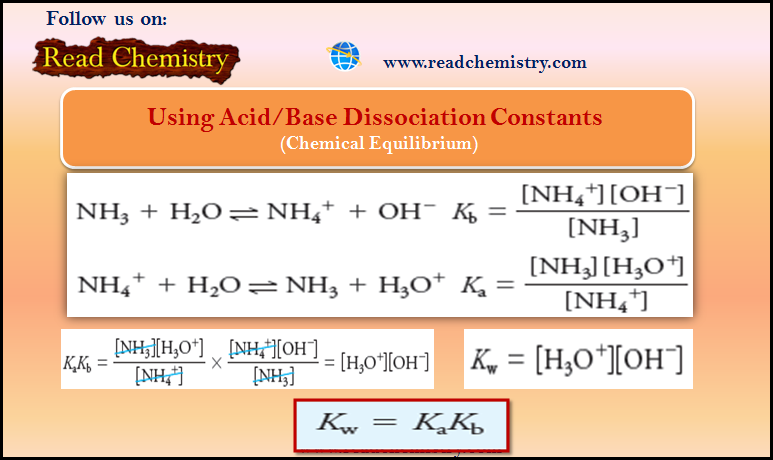
Calculating the pH of Weak Acid and Base Solutions
– In this subject, we will discuss Calculating the pH of Weak Acid and Base…
Read More » -
-
-
-
-
-
-
-
-
-
-
Online MCQ
First law of thermodynamics – MCQ online test
Online MCQ test on First law of thermodynamics – In this topic we offer you,…
Read More » -
-
-
-
-
-
-
-
Free book
Physical Chemistry book , 3rd edition by Robert G. Mortimer
– In this subject, we will discuss free download of Physical Chemistry book, 3rd edition…
Read More » -
-
-
-
-