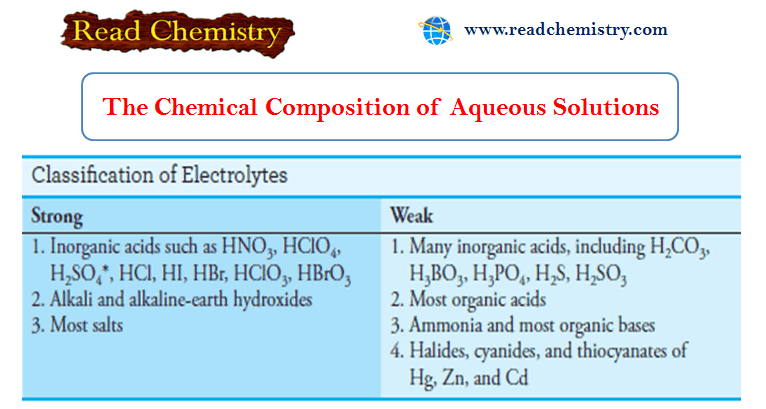
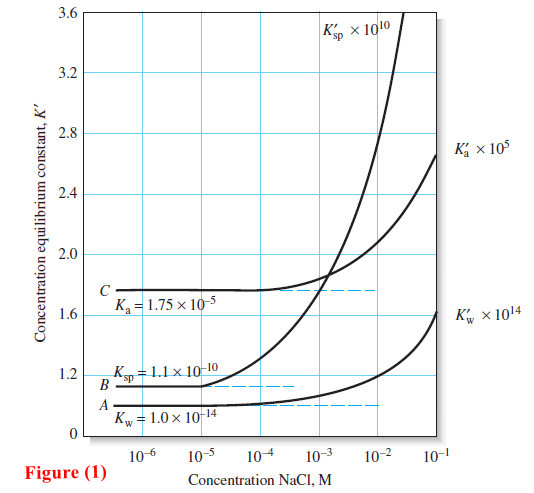
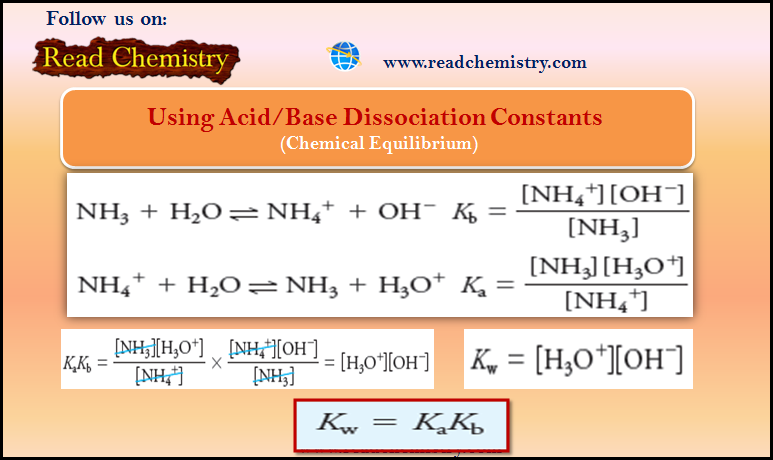
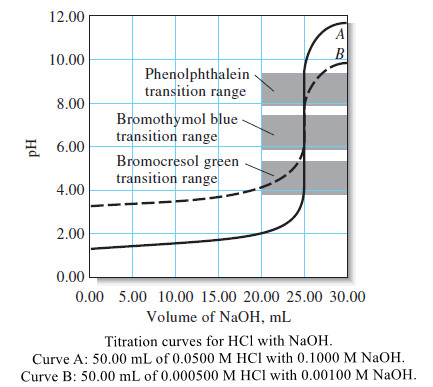
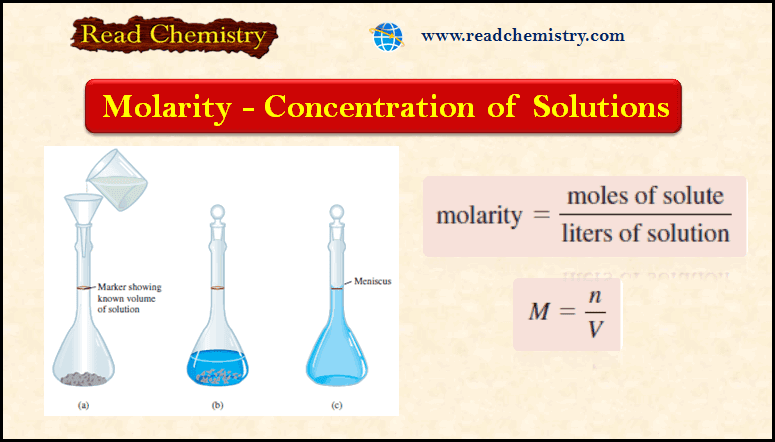
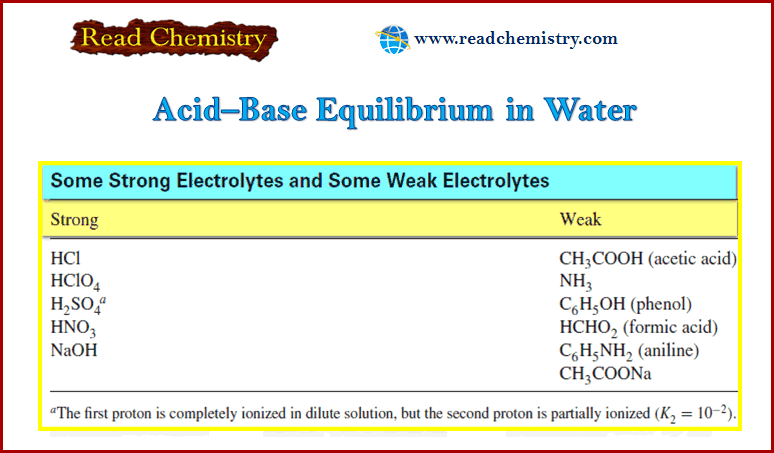
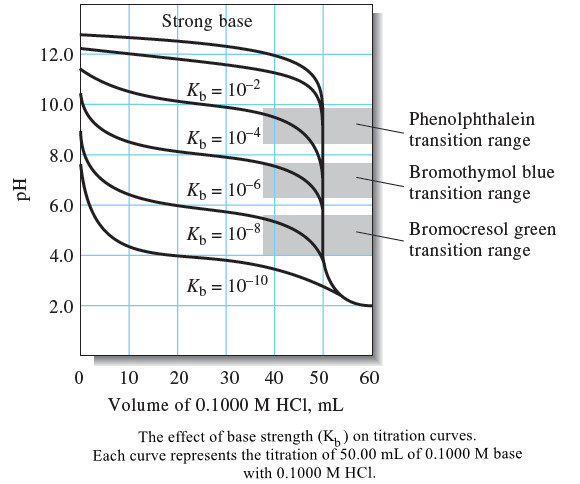
Popular Posts
-
Physical Chemistry
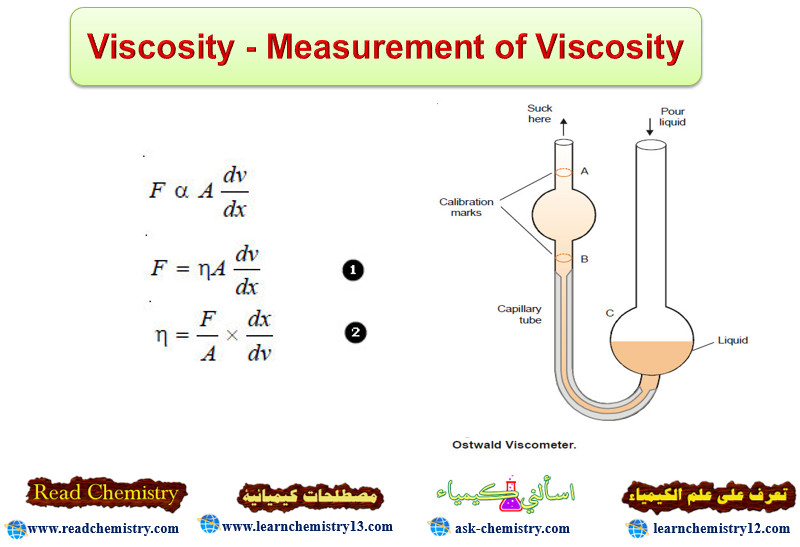
Viscosity – Measurement of Viscosity
Viscosity – Viscosity is the resistance of a liquid to flow. – A liquid may be considered to be consisting…
Read More » -
Physical Chemistry
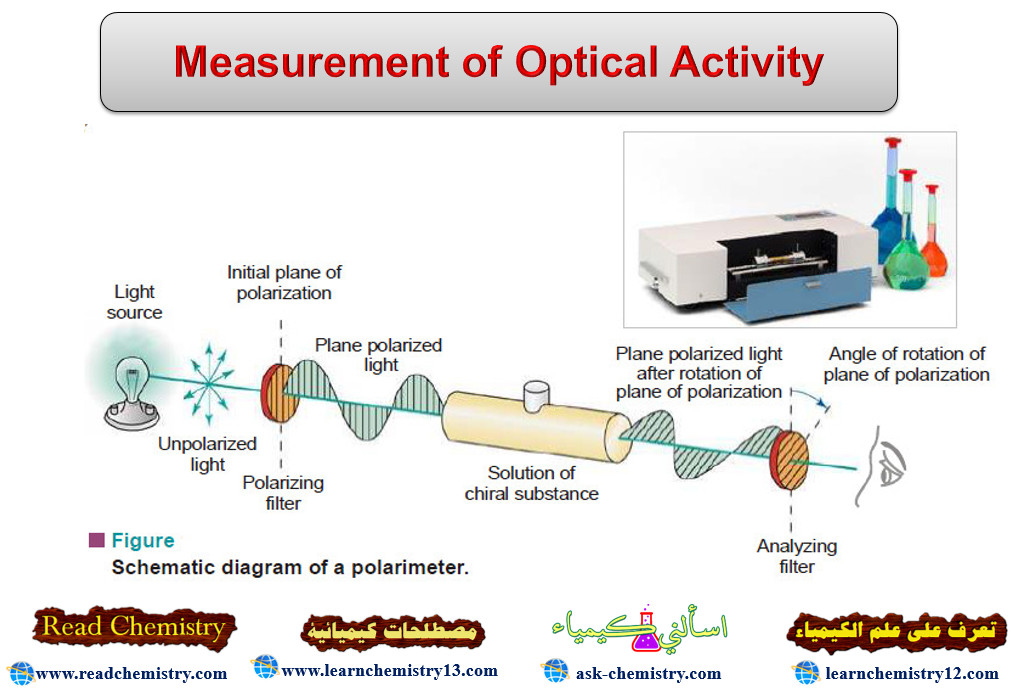
Measurement of Optical Activity
Optical Activity – Optical activity is one of imortant physcial properties of liqiuds – A beam of ordinary light consists…
Read More » -
Analytical Chemistry
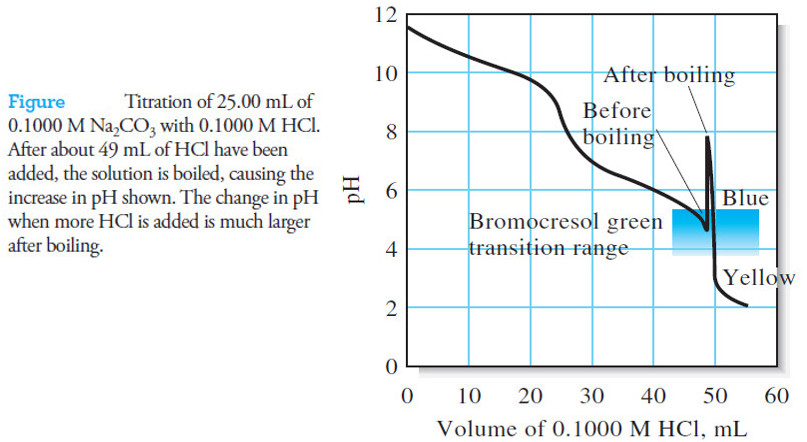
Reagents for Neutralization Titrations
– In this subject, we will discuss the Reagents for Neutralization Titrations. Reagents for Neutralization Titrations – we noted before…
Read More » -
General Chemistry
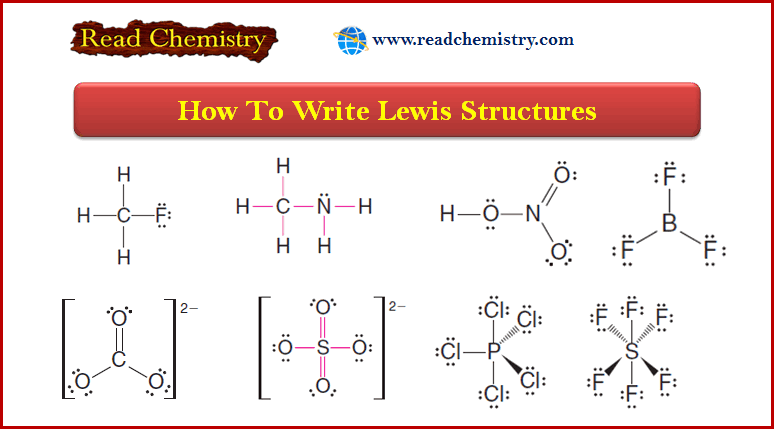
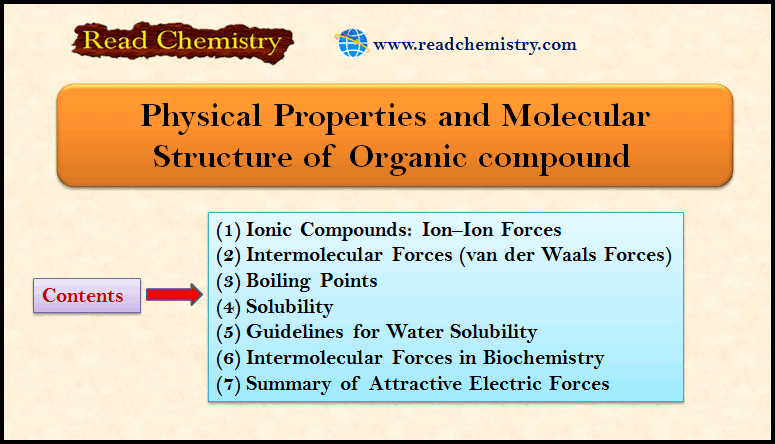
Lewis Structures: Definition, Structural Formula, Examples
– In this subject, we will discuss the Lewis Structures: Definition, Overview, Structural Formula, Examples Definition of Lewis structures –…
Read More » -
Analytical Chemistry
Filtration and Ignition of Solids
– In this subject, we will discuss the Filtration and Ignition of Solids. – Several techniques and experimental arrangements allow…
Read More » -
Organic Chemistry
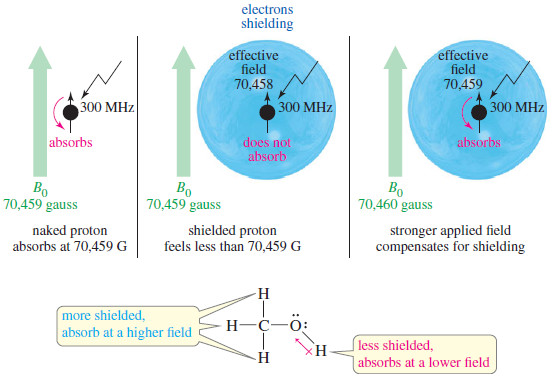
Chemical Shift in NMR Spectroscopy
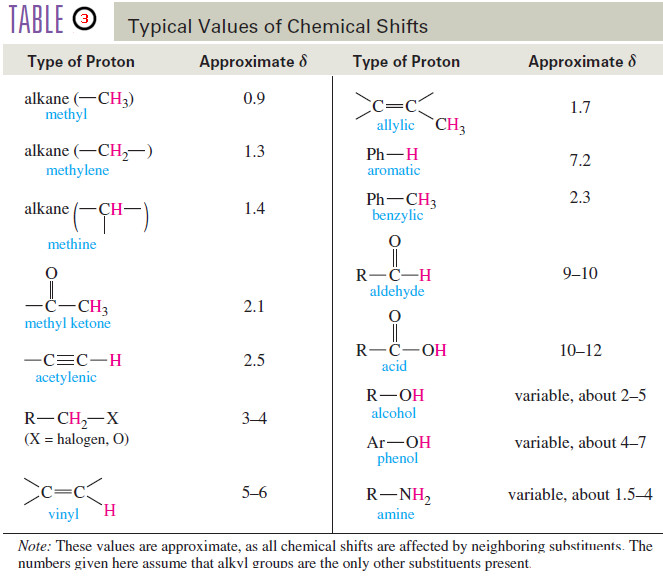
– In this topic, we will discuss the Chemical Shift in 1H NMR Spectroscopy. What is Chemical Shift? – The…
Read More »
-
Organic Chemistry
Ring Opening of Epoxides
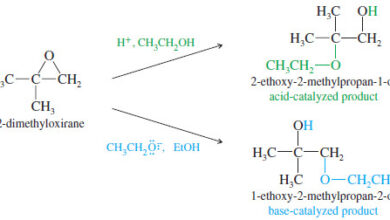
– In this topic, we will talk aboutAcid-Catalyzed Ring Opening of Epoxides, Base-Catalyzed Ring Opening…
Read More » -
-
-
-
-
-
-
-
-
-
-
Online MCQ
First law of thermodynamics – MCQ online test
Online MCQ test on First law of thermodynamics – In this topic we offer you,…
Read More » -
-
-
-
-
-
-
-
-
-
-
General Chemistry
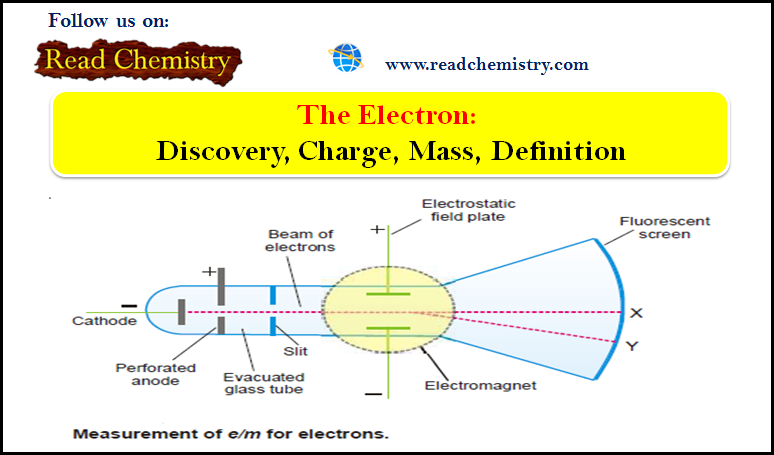
Electron: Discovery, Charge, Mass, Definition
Cathode Rays – The discovery of electron – The knowledge about the electron was derived…
Read More » -
-
-
-
-
-
-
-
-
-
-
Analytical Chemistry
Calibration of Volumetric Glassware in the laboratory
– In this subject, we will discuss How to Calibrate Volumetric Glassware in the laboratory…
Read More » -
-
-
-
-
-
-
-
-
-
-
Online MCQ
First law of thermodynamics – MCQ online test
Online MCQ test on First law of thermodynamics – In this topic we offer you,…
Read More » -
-
-
-
-
-
-
-
Free book
Physical Chemistry book , 3rd edition by Robert G. Mortimer
– In this subject, we will discuss free download of Physical Chemistry book, 3rd edition…
Read More » -
-
-
-
-