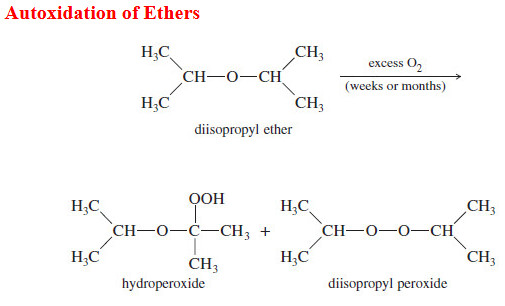
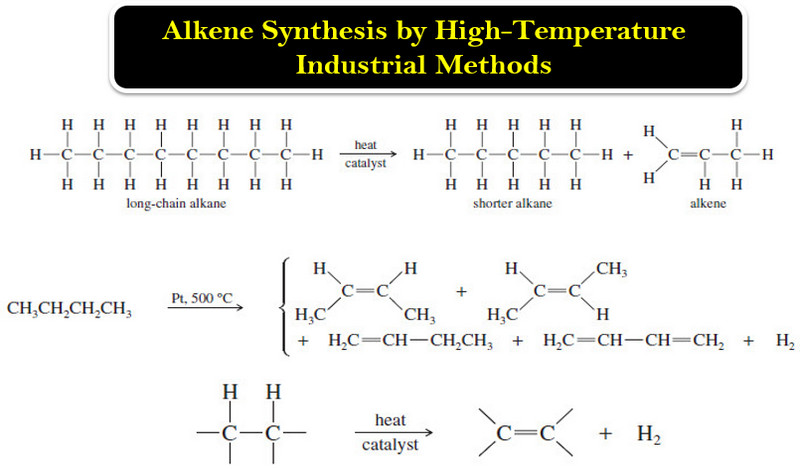
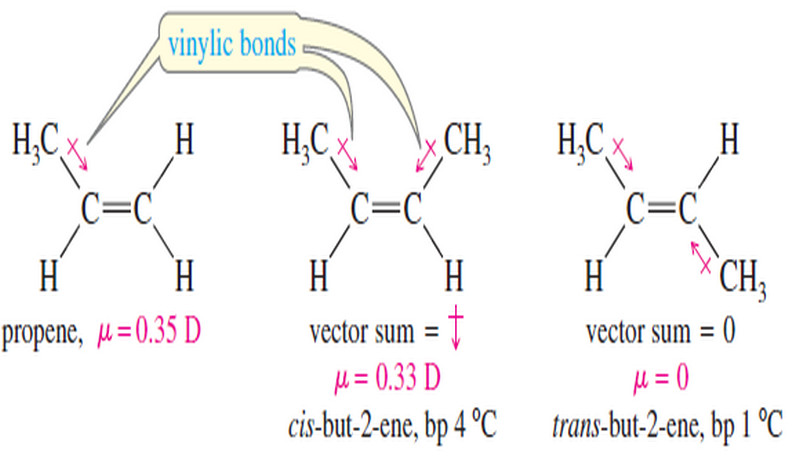
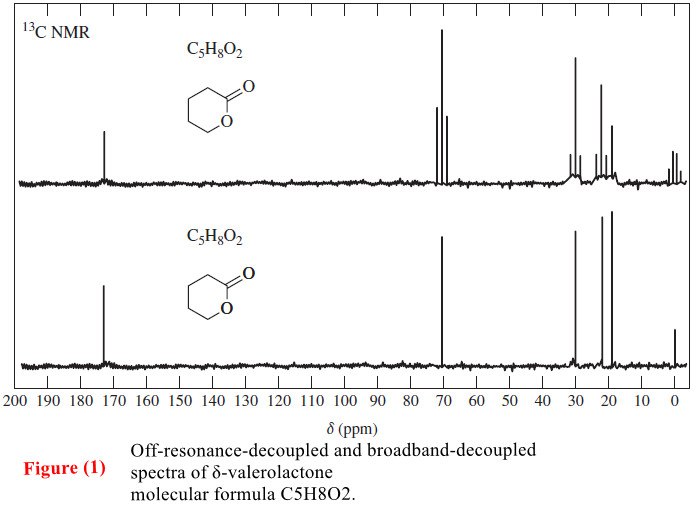
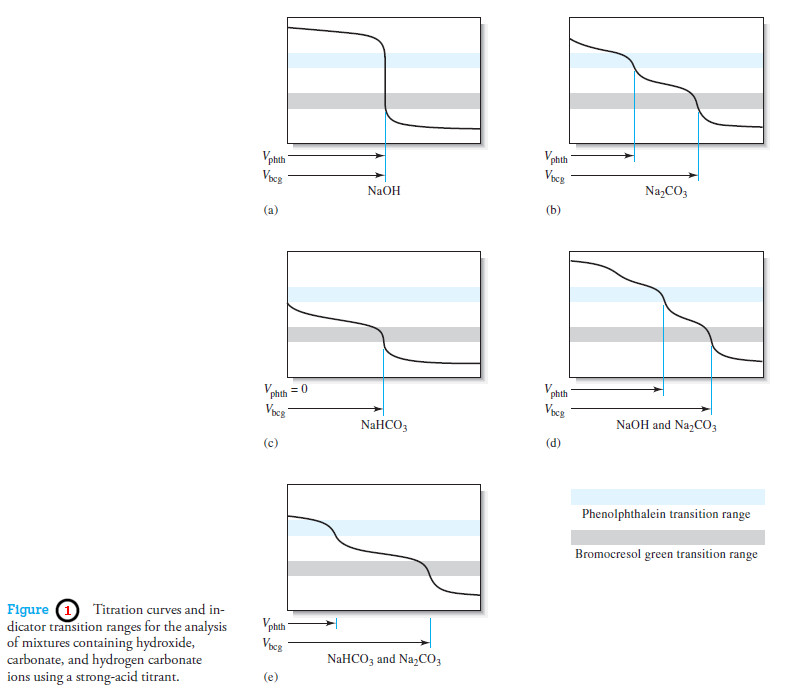
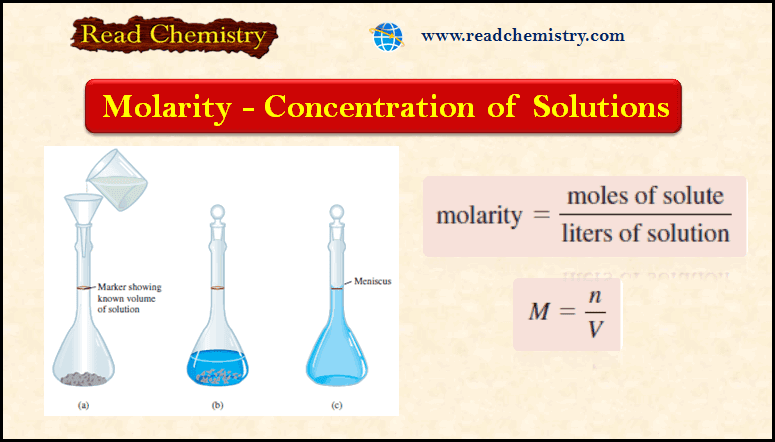
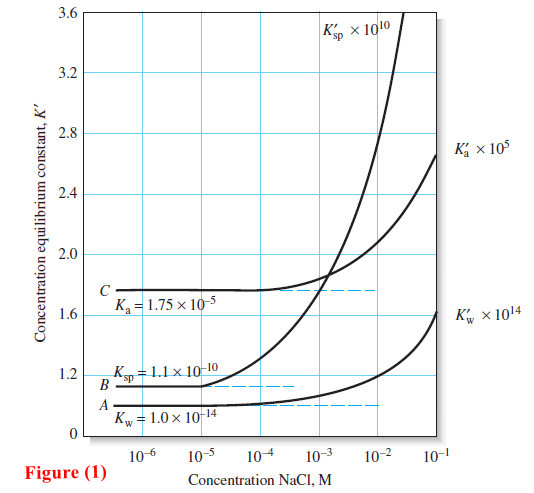
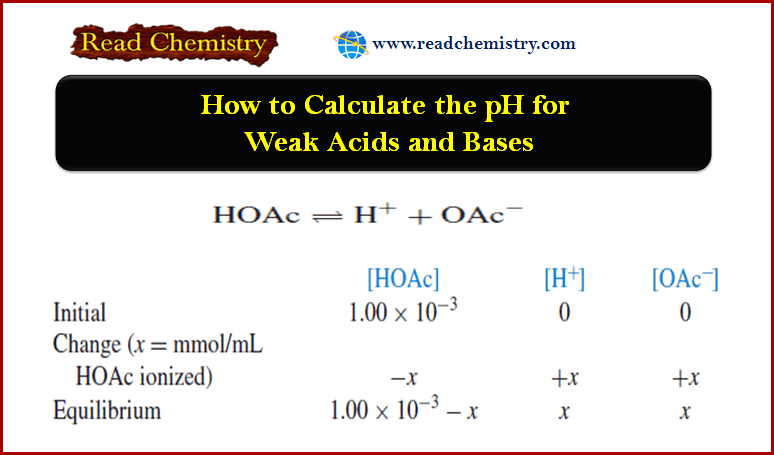
Popular Posts
-
Physical Chemistry
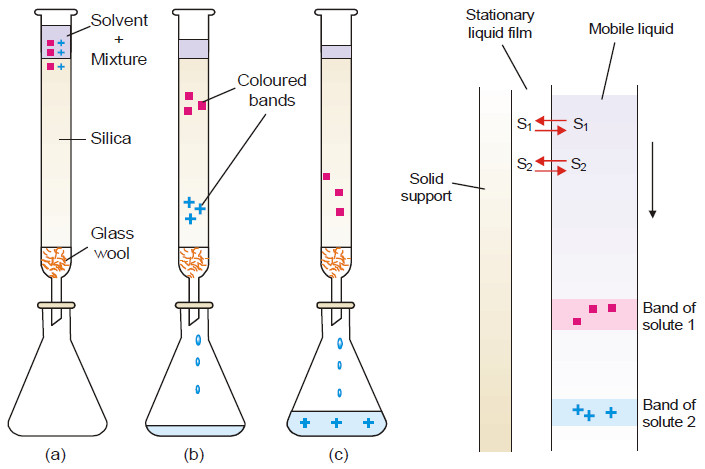
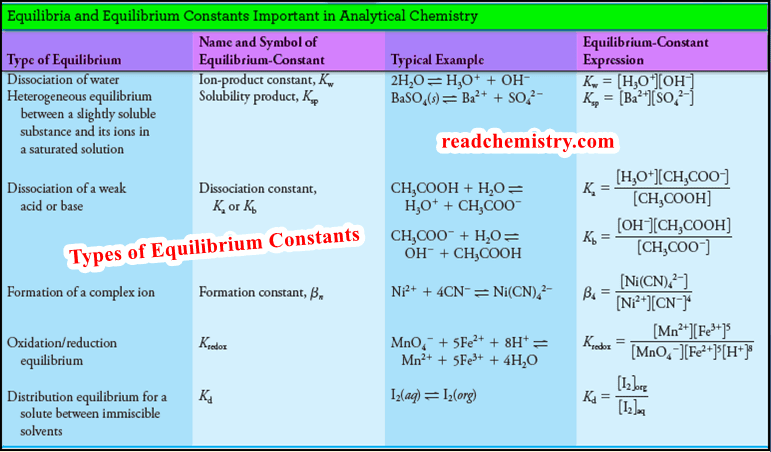
Applications of distribution law
Applications of distribution law – There are numerous applications of distribution law in the laboratory as well as in industry.…
Read More » -
General Chemistry
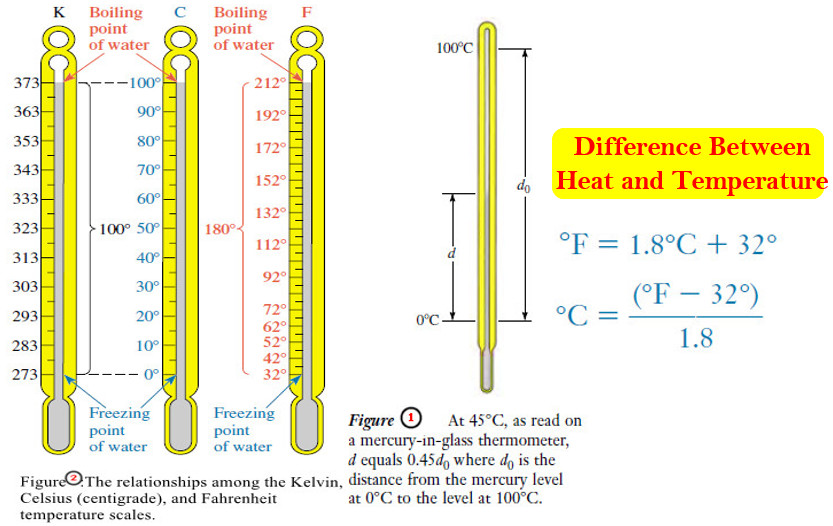
Heat and Temperature
Difference Between Heat and Temperature – Heat is one form of energy. – Many forms of energy can be interconverted…
Read More » -
Physical Chemistry
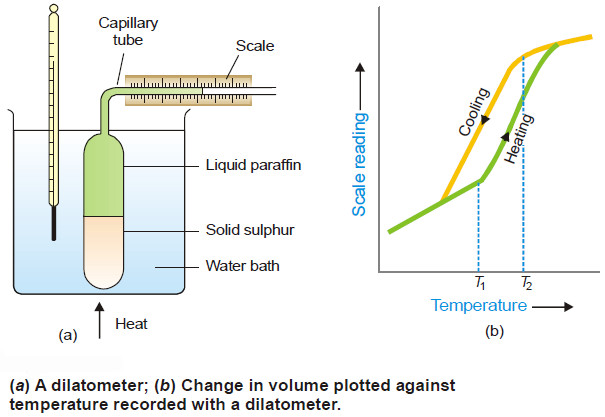
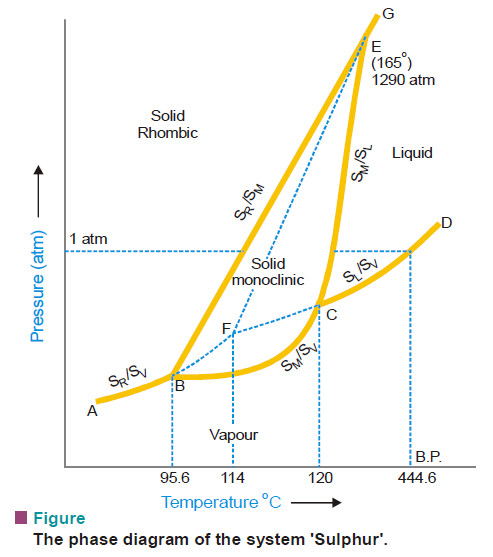
Polymorphism – Allotropy
Polymorphism – The occurrence of the same substance in more than one crystalline forms is known as Polymorphism. – Polymorphism…
Read More » -
Analytical Chemistry
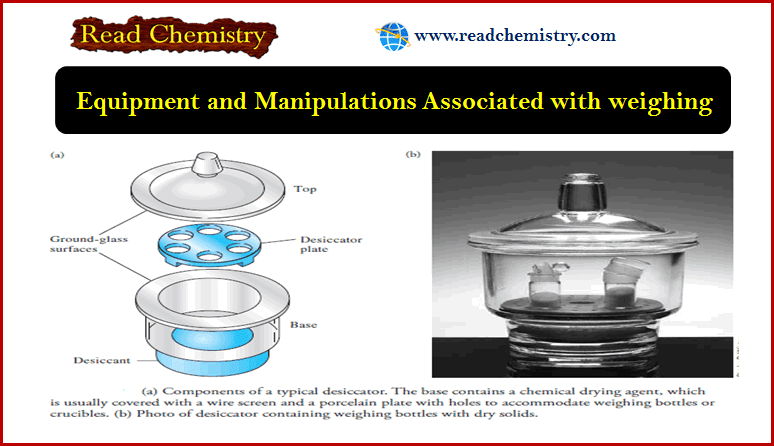
Safety in the laboratory
– In this subject, we will discuss the Safety in the laboratory Safety in The Laboratory – There is necessarily…
Read More » -
Physical Chemistry
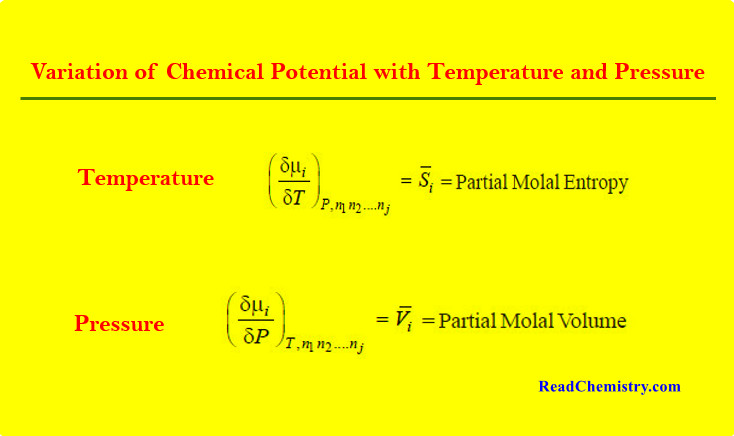
Chemical Potential
– In this topic, we will discuss The Chemical Potential and Variation of Chemical Potential with Temperature and Pressure. Partial…
Read More » -
Physical Chemistry
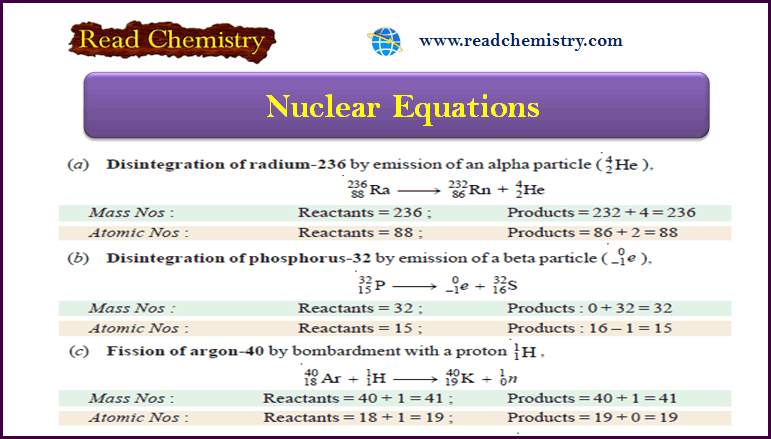
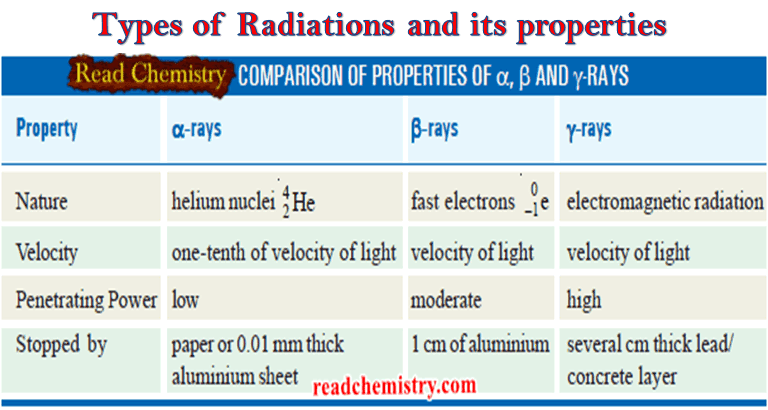
Nuclear Equations: Balancing, Rules, Practice
– In this subject, we will discuss the Nuclear Equations: Balancing, Rules, Practice Nuclear Equations – Similar to a chemical…
Read More »
-
Organic Chemistry
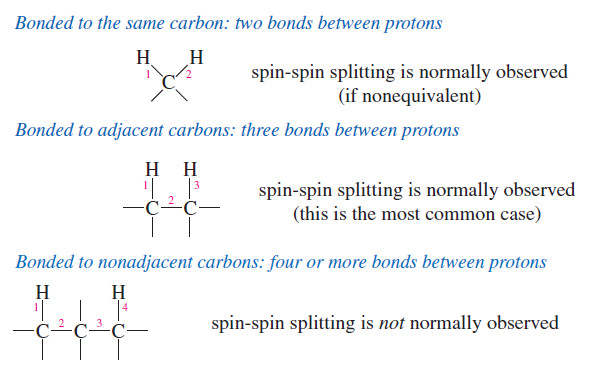
Spin-Spin Splitting in ¹H NMR Spectra
– In this topic, we will discuss The Spin-Spin Splitting in ¹H NMR Spectra. Theory…
Read More » -
-
-
-
-
-
-
-
-
-
-
Physical Chemistry
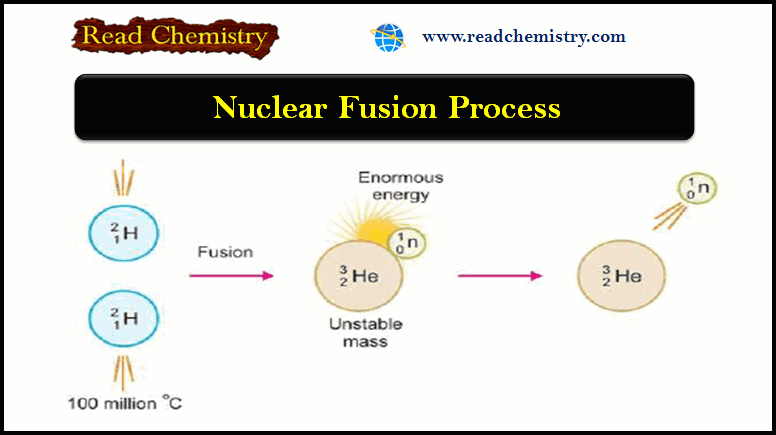
Nuclear Fusion: Definition, Occurrence, Examples, Applications
– In this subject, we will discuss the Nuclear Fusion Process ( Definition, Occurrence, Examples,…
Read More » -
-
-
-
-
-
-
-
-
-
-
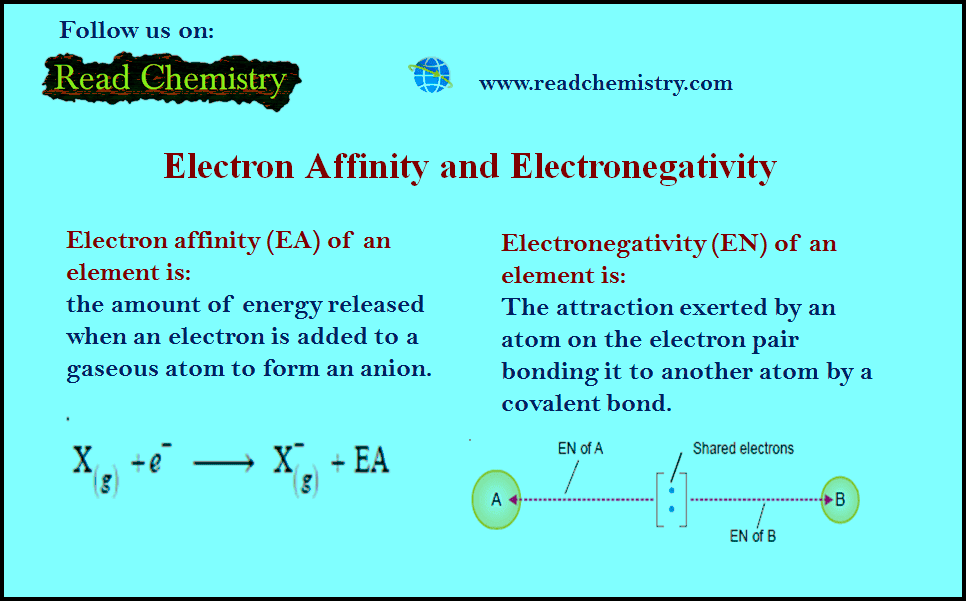
General Chemistry
Electronegativity and Electron Affinity
– In this subject, we will discuss the difference Between Electronegativity and Electron Affinity Electron…
Read More » -
-
-
-
-
-
-
-
-
-
-
Analytical Chemistry
Filtration and Ignition of Solids
– In this subject, we will discuss the Filtration and Ignition of Solids. – Several…
Read More » -
-
-
-
-
-
-
-
-
-
-
Online MCQ
First law of thermodynamics – MCQ online test
Online MCQ test on First law of thermodynamics – In this topic we offer you,…
Read More » -
-
-
-
-
-
-
-
Free book
Physical Chemistry book , 3rd edition by Robert G. Mortimer
– In this subject, we will discuss free download of Physical Chemistry book, 3rd edition…
Read More » -
-
-
-
-