Popular Posts
-
Analytical Chemistry
Concentration of Solutions: Definitions, Formulas, Solved Problems
– In this subject, we will discuss the Concentration of Solutions Concentration of Solutions (Definitions, Formulas, Solved Problems). Concentration of…
Read More » -
Organic Chemistry
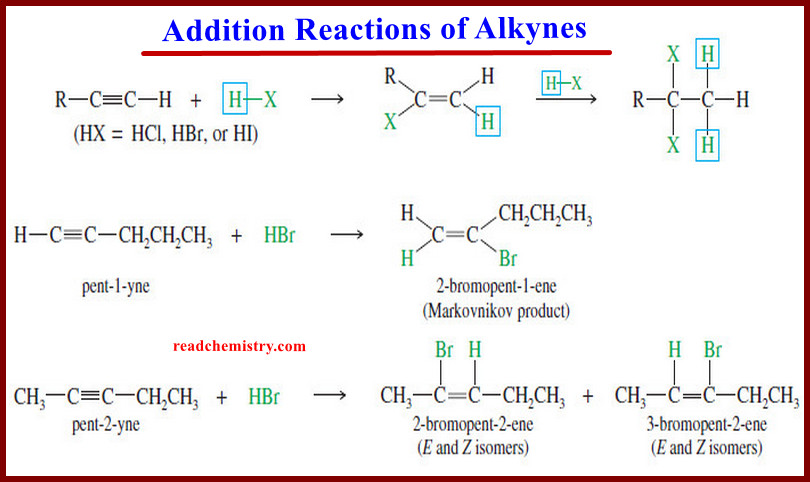
Addition Reactions of Alkynes
Addition Reactions of Alkynes – Many of the reactions of alkynes are similar to the corresponding reactions of alkenes because…
Read More » -
Physical Chemistry
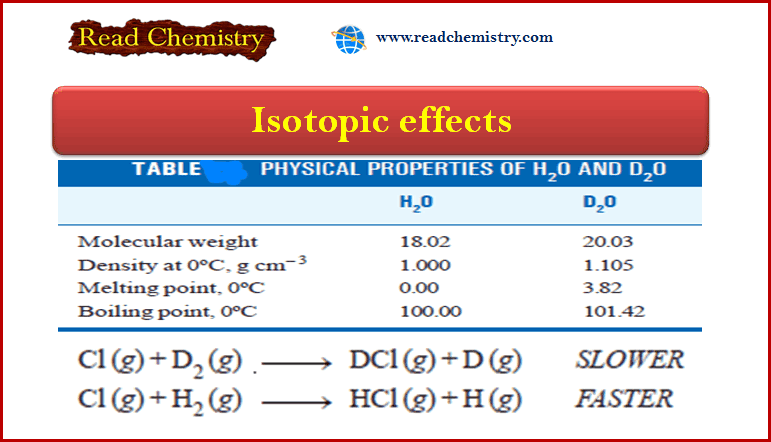
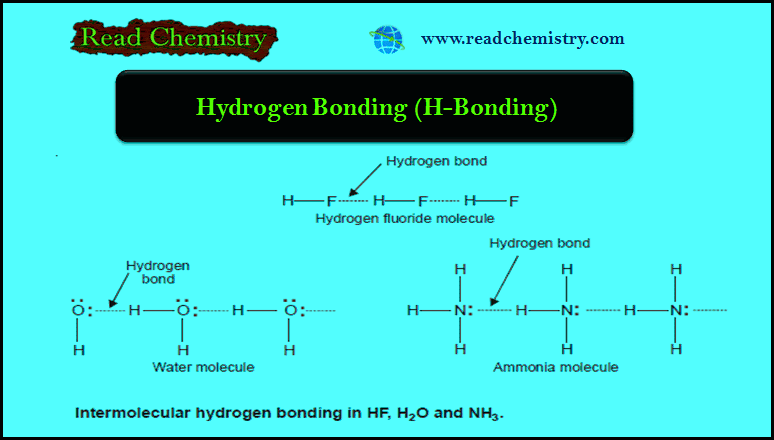
Isotopic effects: Definition, Applications
– In this subject, we will discuss the Isotopic effects: definition, Applications Definition of isotopes Isotopes may be defined as…
Read More » -
Physical Chemistry
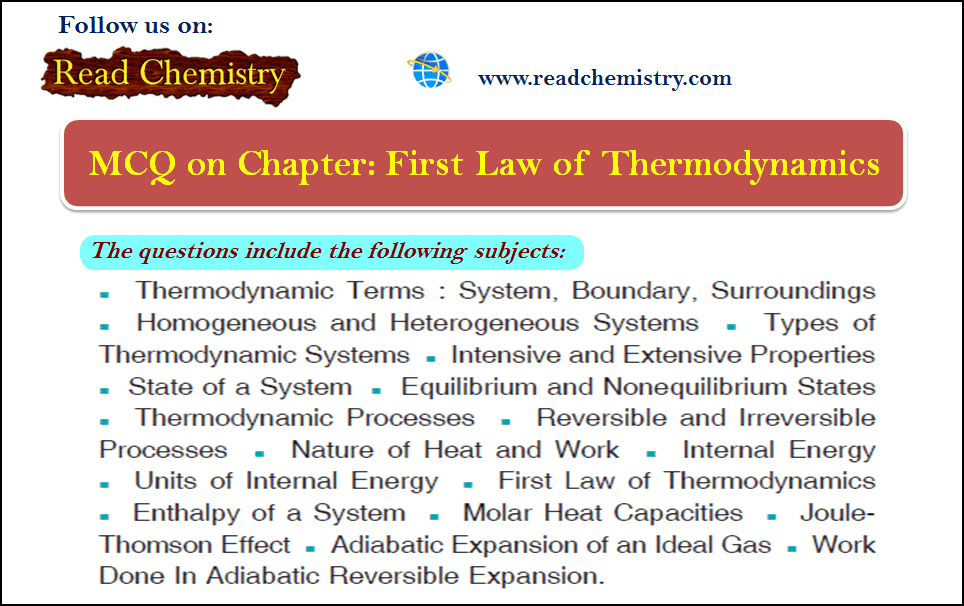
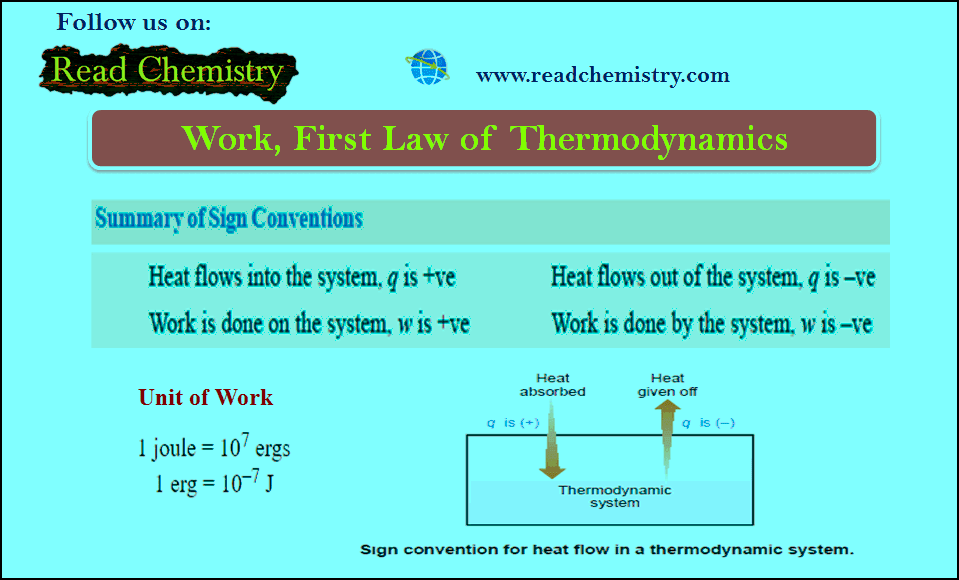
MCQ on the First law of Thermodynamics
MCQ on the First Law of Thermodynamics – In this subject, you will find 50 questions and answers MCQ on…
Read More » -
Organic Chemistry
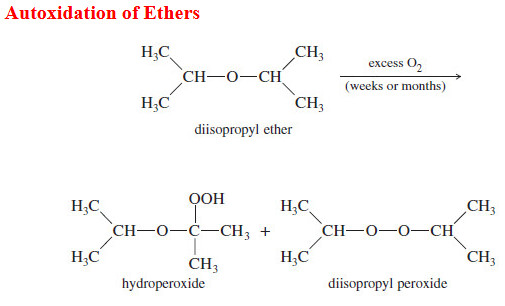
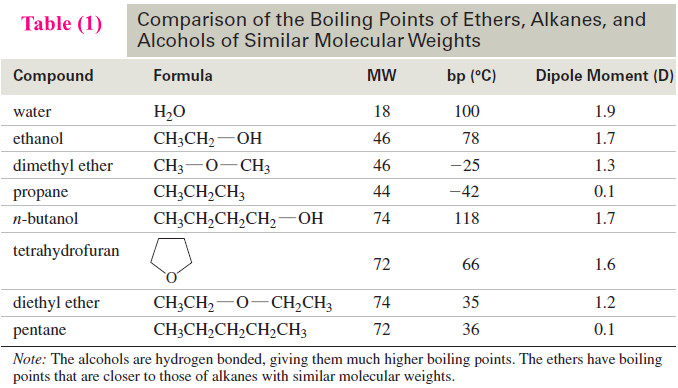
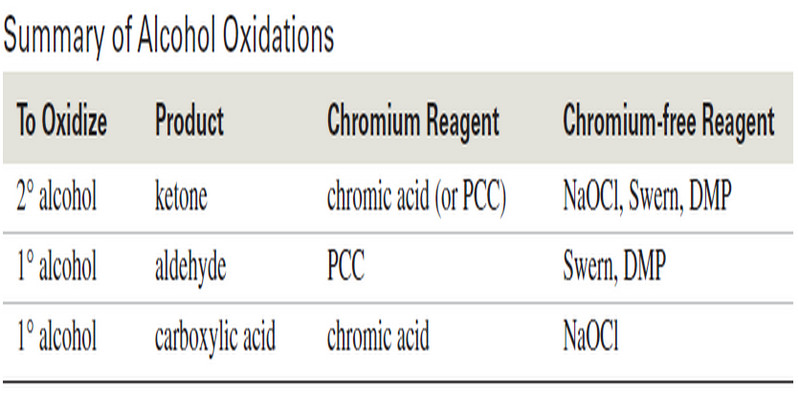
Autoxidation of Ethers
– In this topic, we will discuss The autoxidation of Ethers. What are Ethers? Ethers are compounds of formula R-O-R,…
Read More » -
Physical Chemistry
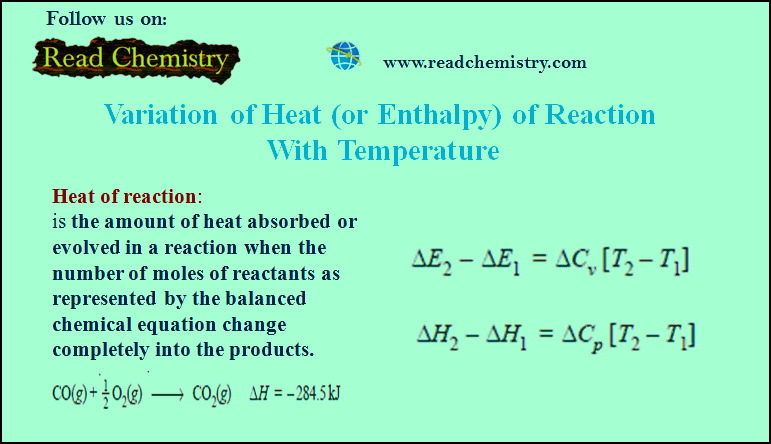
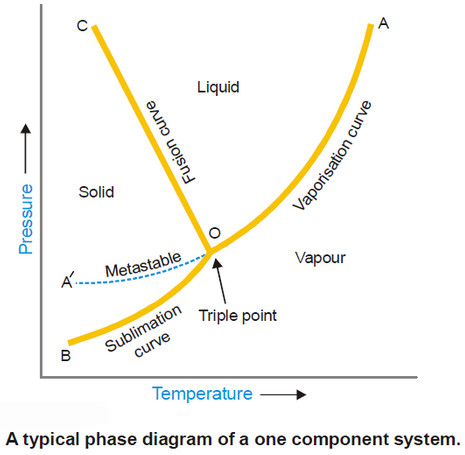
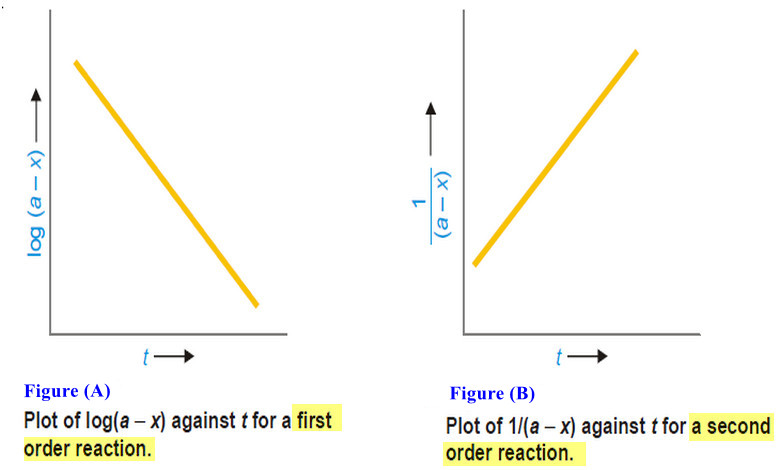
Variation of heat of reaction with temperature
– In this subject, the Variation of heat of reaction with temperature will be discussed. Heat of Reaction or Enthalpy…
Read More »
-
Organic Chemistry
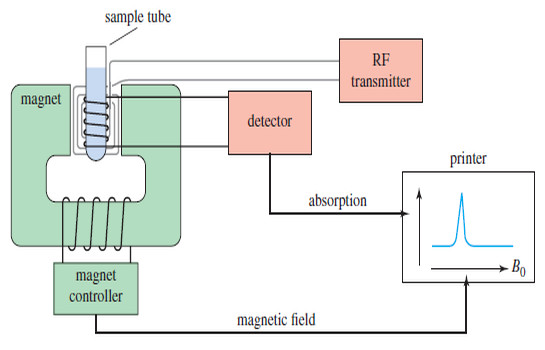
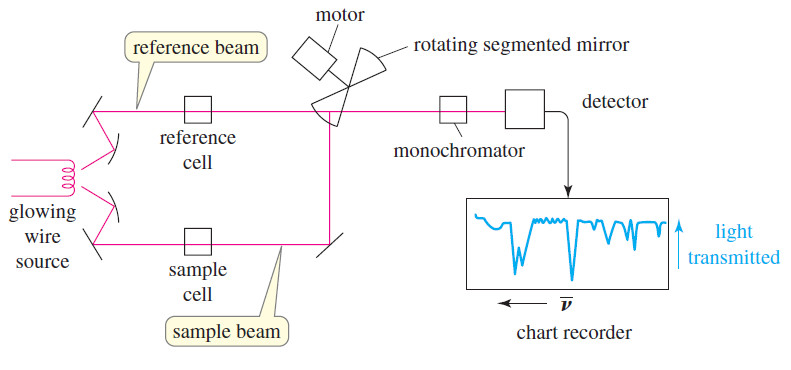
NMR spectrometer
What happens in an NMR spectrometer? – Before discussing the design of spectrometers, let’s review…
Read More » -
-
-
-
-
-
-
-
-
-
-
Physical Chemistry
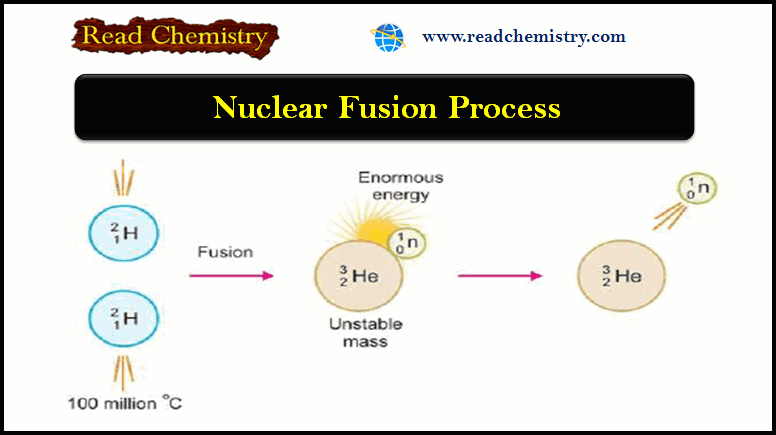
Nuclear Fusion: Definition, Occurrence, Examples, Applications
– In this subject, we will discuss the Nuclear Fusion Process ( Definition, Occurrence, Examples,…
Read More » -
-
-
-
-
-
-
-
-
-
-
General Chemistry
Ions and ionic compounds
– In this topic, we will discuss definition of The Ions and ionic compounds Ions…
Read More » -
-
-
-
-
-
-
-
-
-
-
Analytical Chemistry
The Laboratory Notebook
The Laboratory Notebook – A laboratory notebook is needed to record measurements and observations concerning…
Read More » -
-
-
-
-
-
-
-
-
-
-
Online MCQ
First law of thermodynamics – MCQ online test
Online MCQ test on First law of thermodynamics – In this topic we offer you,…
Read More » -
-
-
-
-
-
-
-
Free book
Physical Chemistry book , 3rd edition by Robert G. Mortimer
– In this subject, we will discuss free download of Physical Chemistry book, 3rd edition…
Read More » -
-
-
-
-