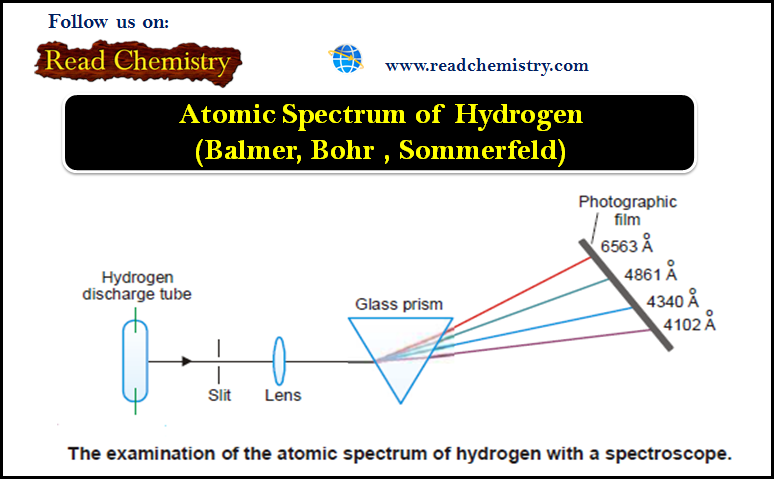
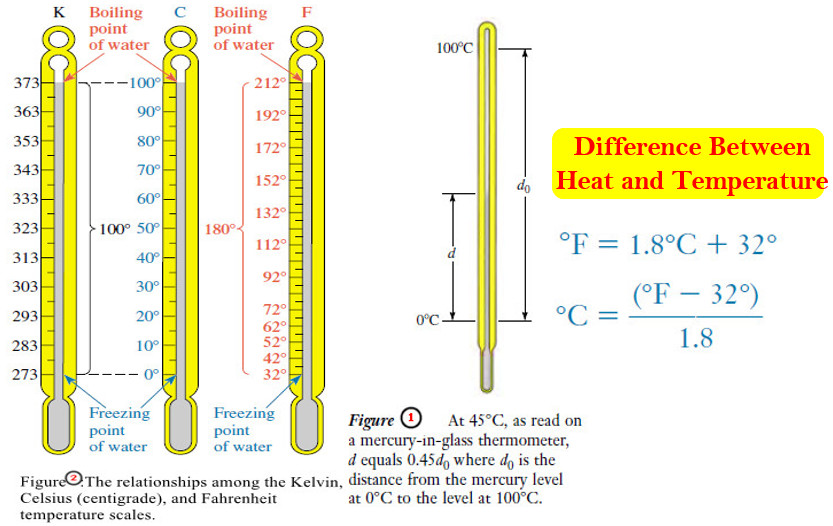
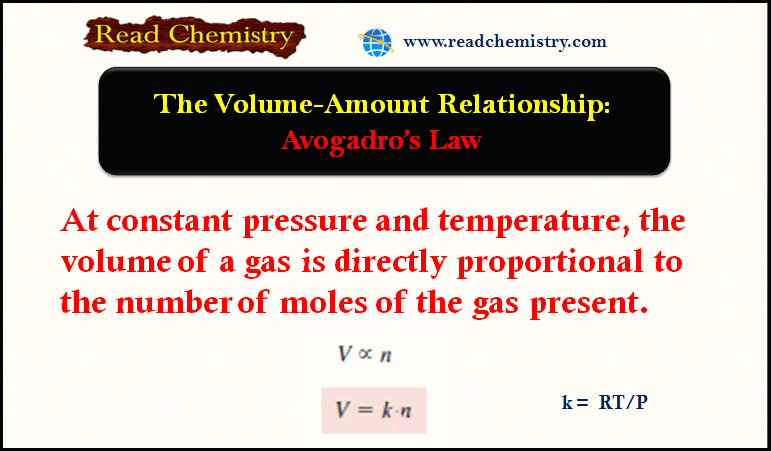
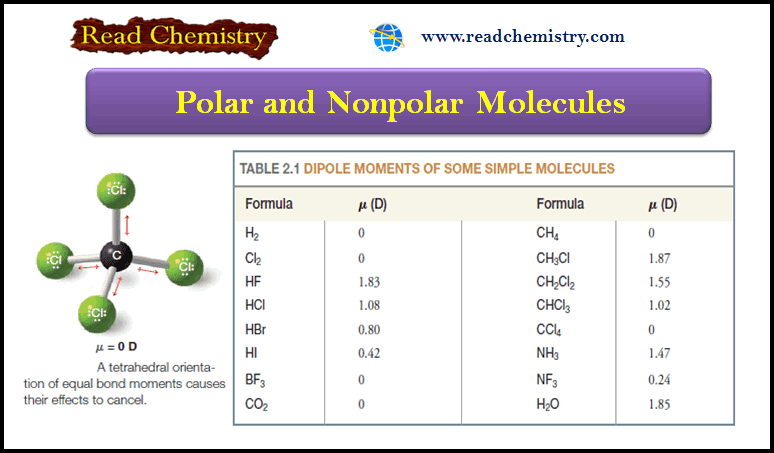
Popular Posts
-
Organic Chemistry
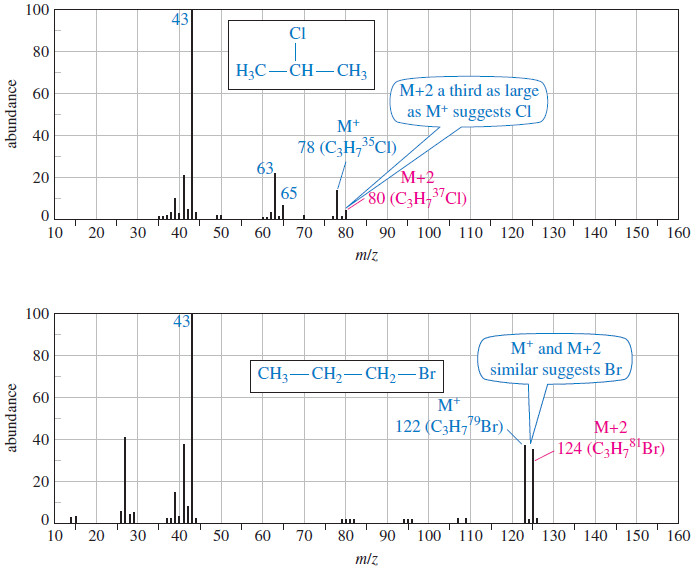
Determination of the Molecular Formula by Mass Spectrometry
Determination of the Molecular Formula by Mass Spectrometry – we can Determine the Molecular Formula by Mass Spectrometry and we…
Read More » -
Analytical Chemistry
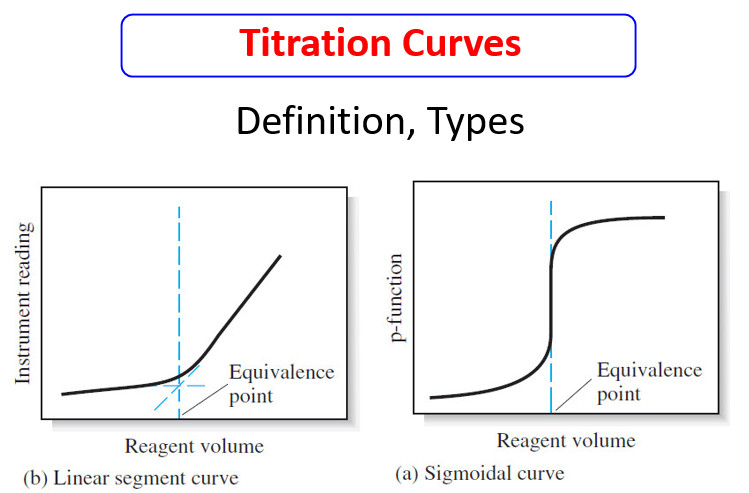
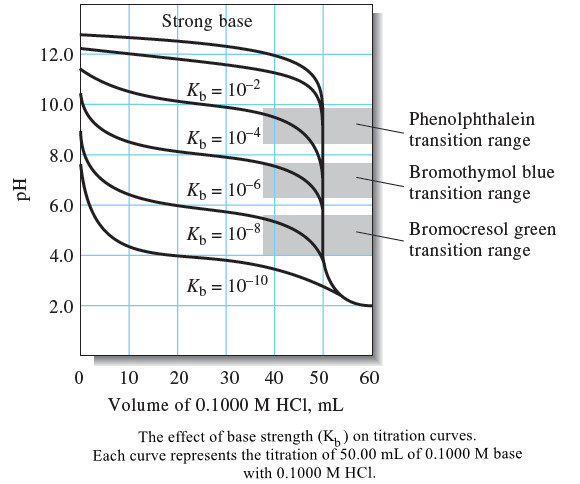
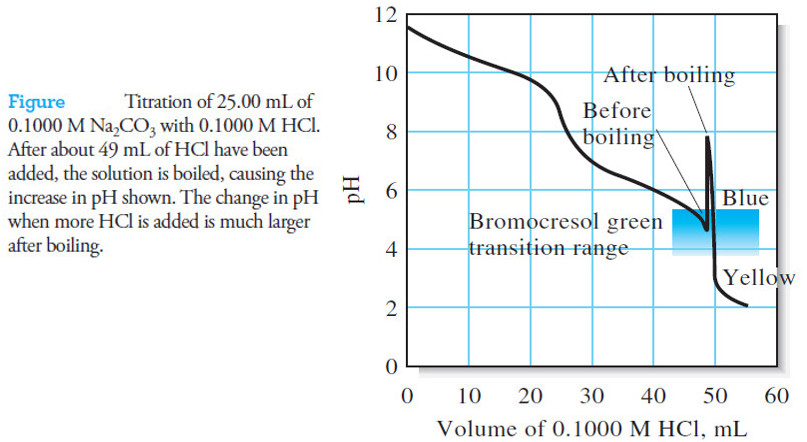
Titration Curves in Analytical Chemistry : Definition, Types
– In this topic, we will discuss the Titration Curves in Analytical Chemistry : Definition and Types Titration Curves –…
Read More » -
Physical Chemistry
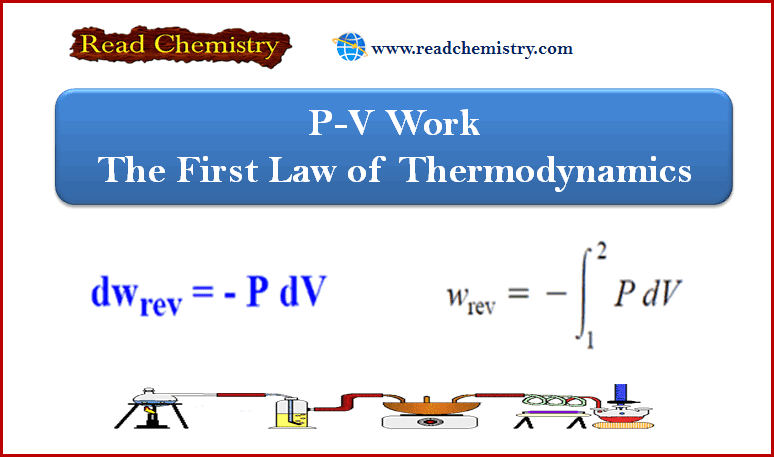
P-V work – The First Law of Thermodynamics
– In this subject, we will discuss Pressure-volume work – P-V work – the First Law of Thermodynamics Work –…
Read More » -
Free book
Fundamentals of Electrochemistry book by V.S. Bagotsky
– In this subject, we will discuss free download of Fundamentals of Electrochemistry book by V.S. Bagotsky The Preface of…
Read More » -
General Chemistry
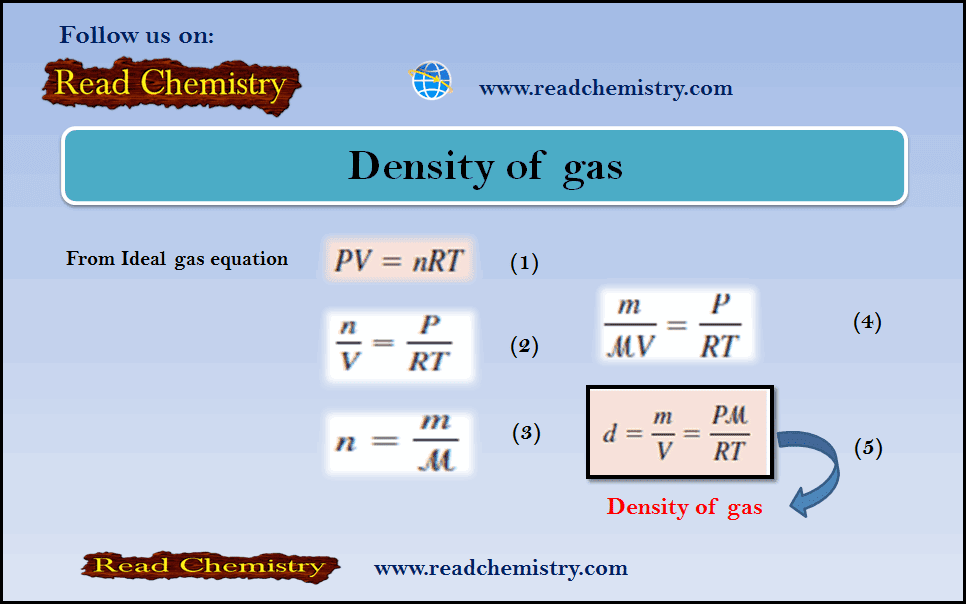
Density of gas: Definition, Equation, Solved Examples
– In this subject, we will discuss the Density of gas (Definition, Equation, Solved Examples) The Density of gas –…
Read More » -
Organic Chemistry
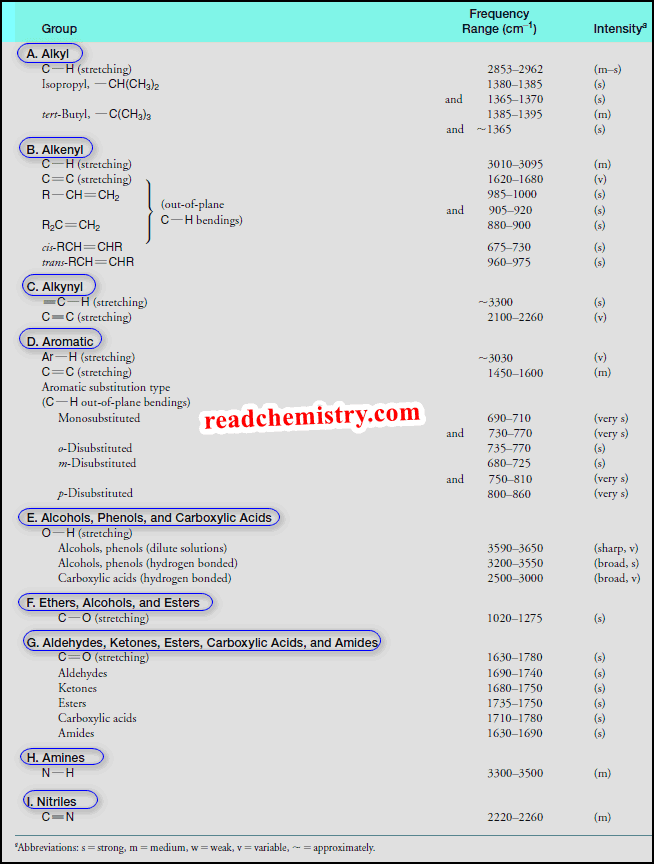
How to interpret IR spectrum without Knowledge of the structure
– In this subject, we will discuss How to interpret an IR spectrum without any Knowledge of the structure How…
Read More »
-
Organic Chemistry
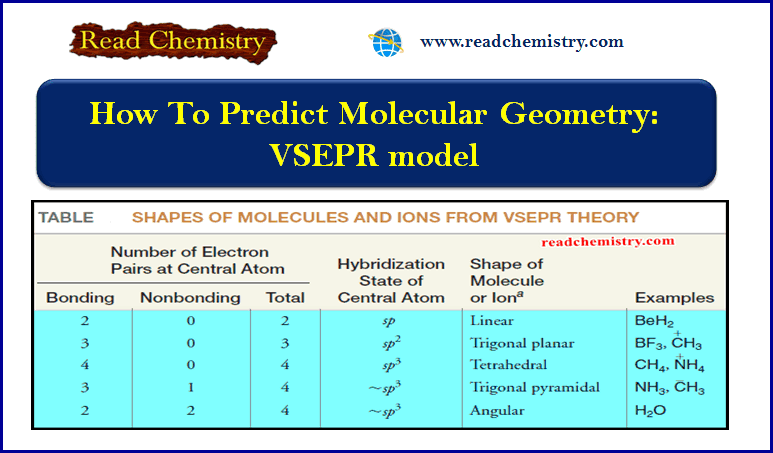
How To Predict Molecular Geometry: VSEPR model
How To Predict Molecular Geometry: VSEPR model ** We can predict the arrangement…
Read More » -
-
-
-
-
-
-
-
-
-
-
Free book
Fundamentals of Chemistry book by Romain Elsair – Free download
– In this subject, we will discuss the free download of Fundamentals of Chemistry book…
Read More » -
-
-
-
-
-
-
-
-
-
-
General Chemistry
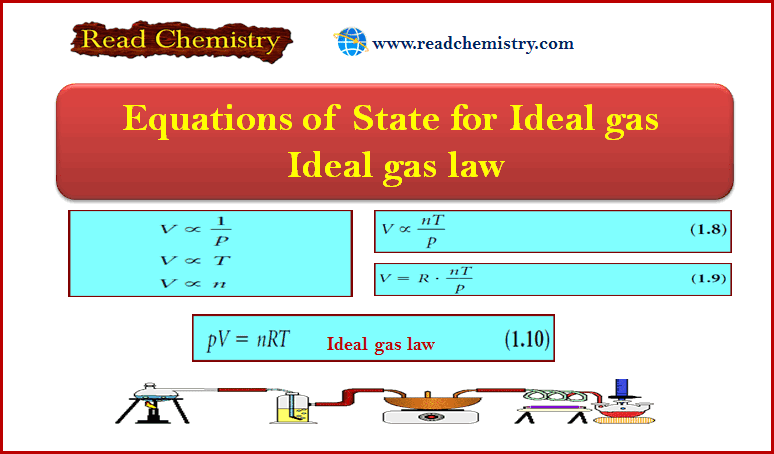
Equations of State for Ideal gas – Ideal gas law
Equations of State for Ideal gas ** Phenomenological thermodynamics is based on experiment, on…
Read More » -
-
-
-
-
-
-
-
-
-
-
Analytical Chemistry
Burets : Overview, Uses, Function, Cleaning
Burets – Burets, like measuring pipets, make it possible to deliver any volume up to…
Read More » -
-
-
-
-
-
-
-
-
-
-
Online MCQ
First law of thermodynamics – MCQ online test
Online MCQ test on First law of thermodynamics – In this topic we offer you,…
Read More » -
-
-
-
-
-
-
-
Free book
Physical Chemistry book , 3rd edition by Robert G. Mortimer
– In this subject, we will discuss free download of Physical Chemistry book, 3rd edition…
Read More » -
-
-
-
-