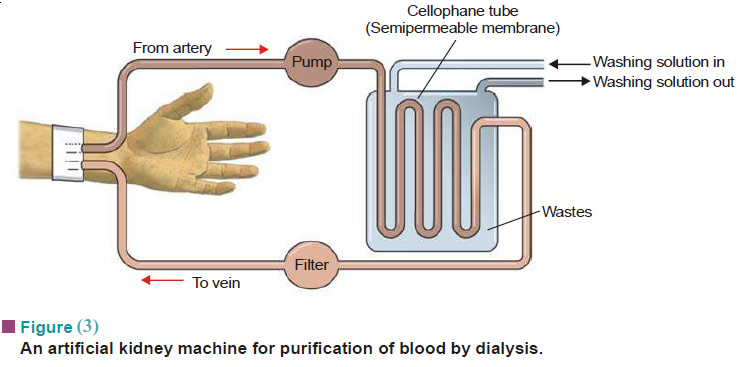
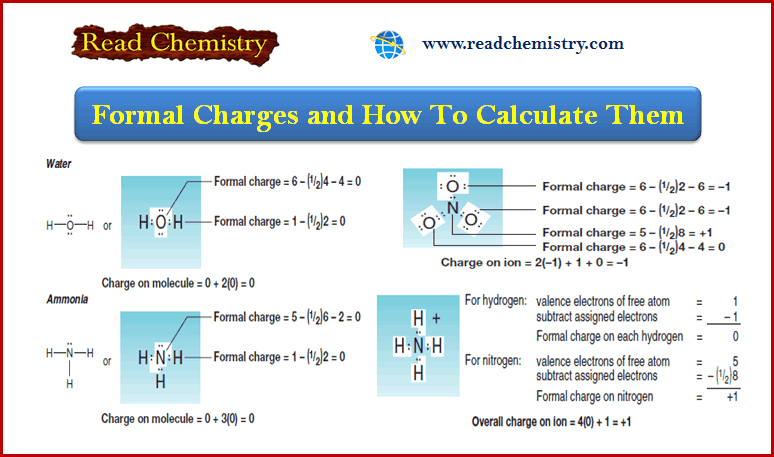
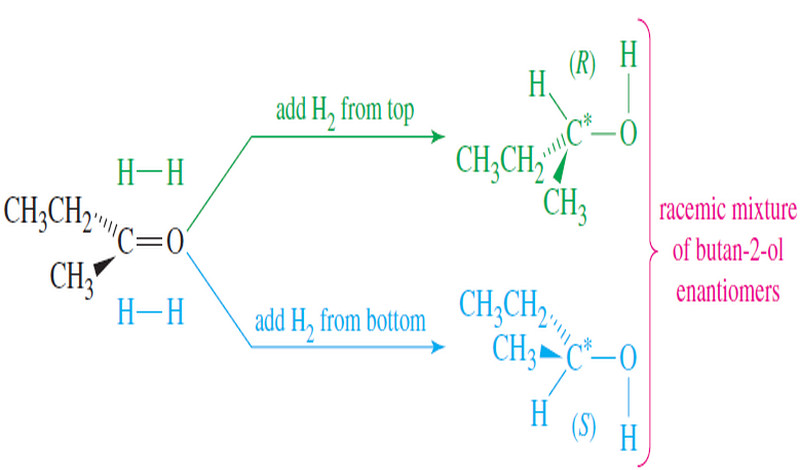
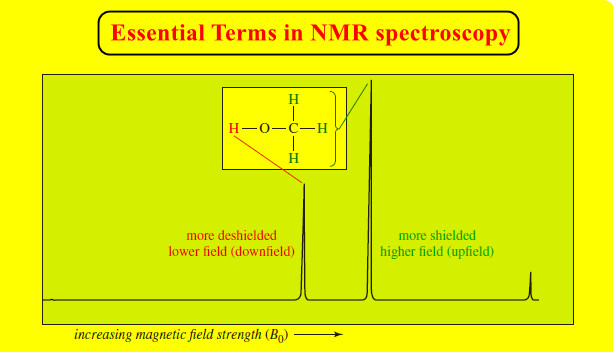
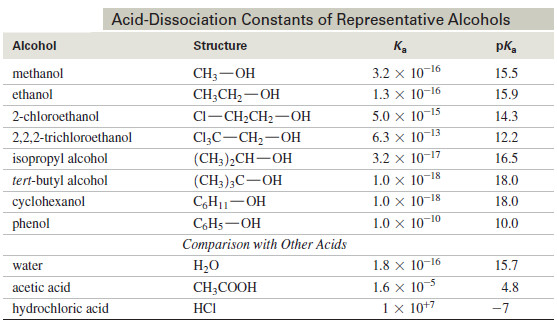
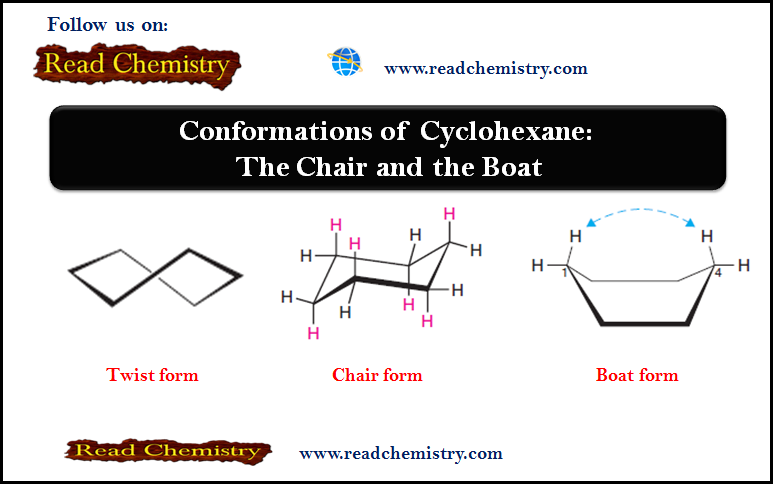
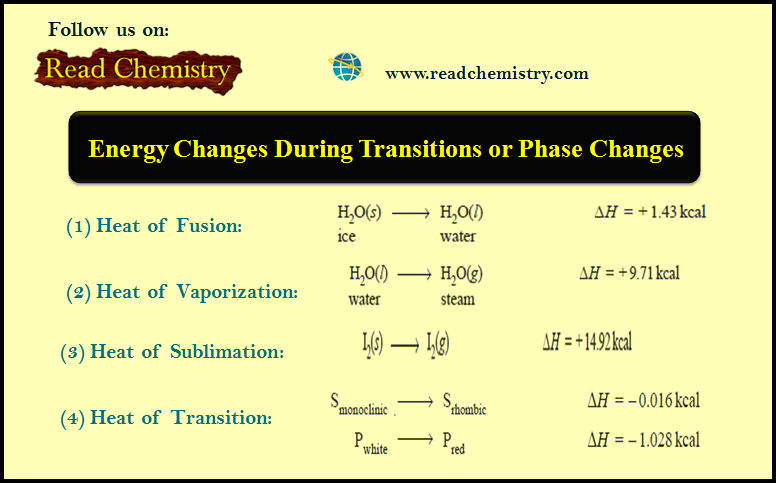
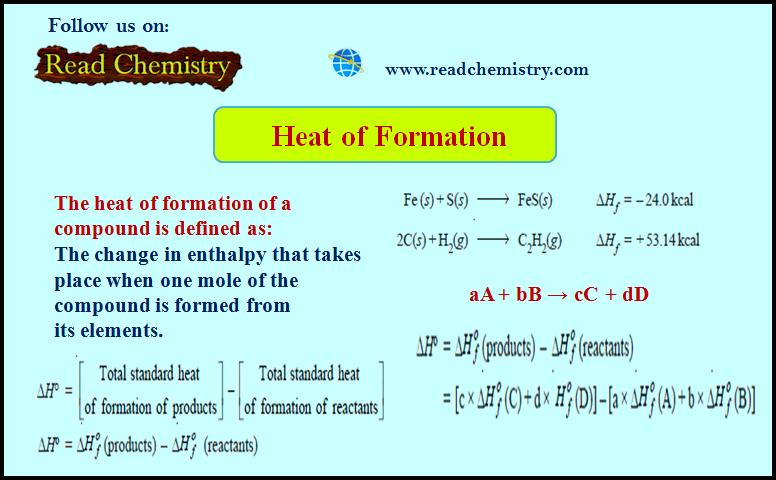
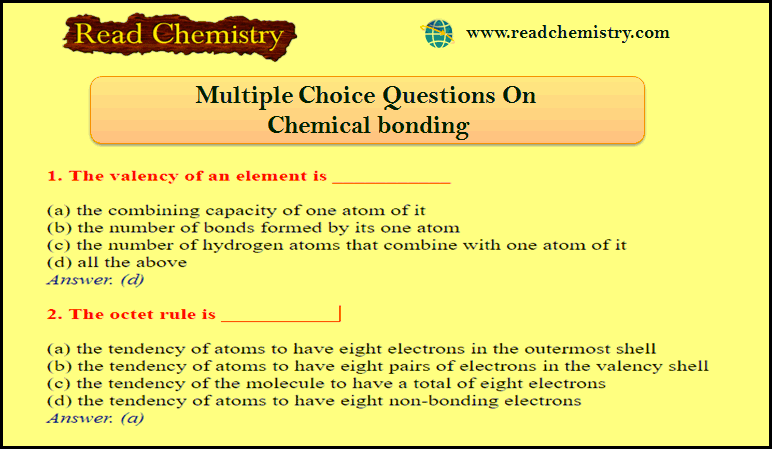
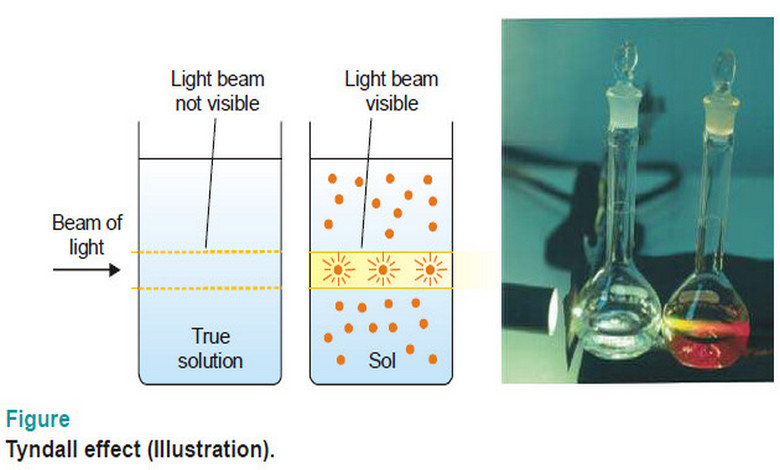
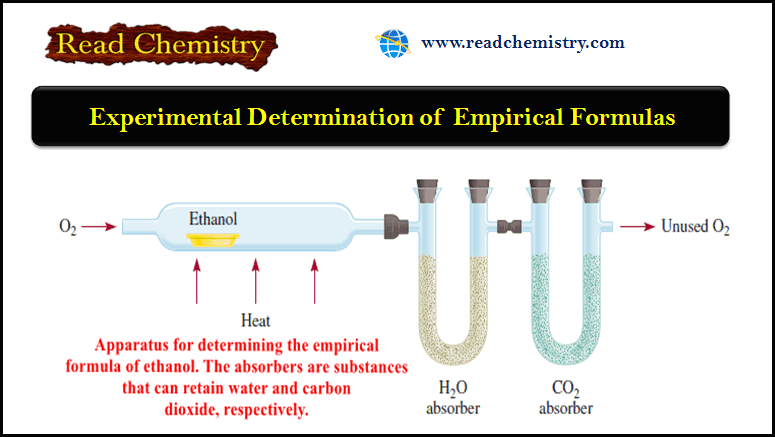
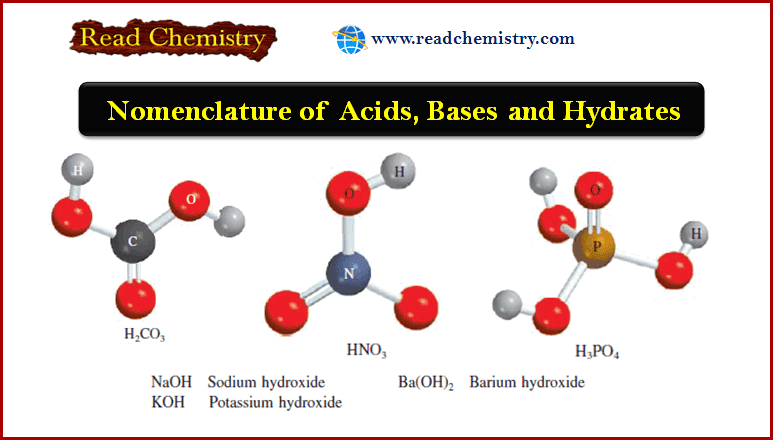
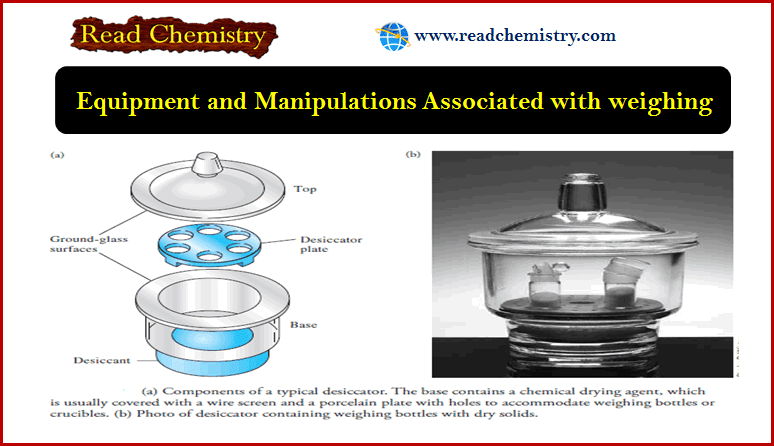
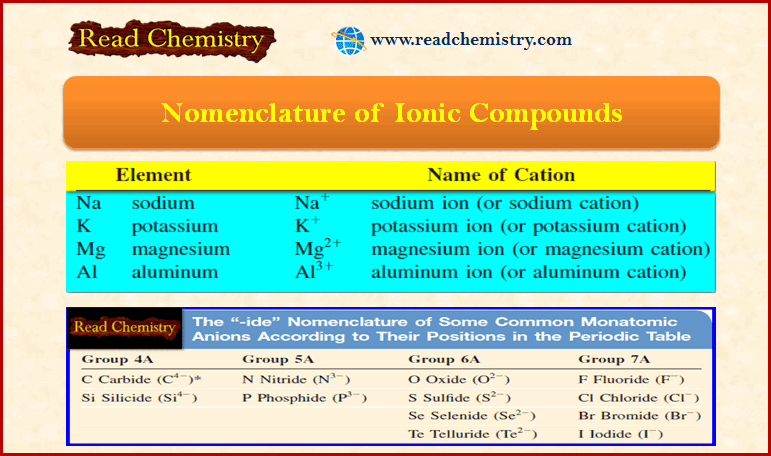
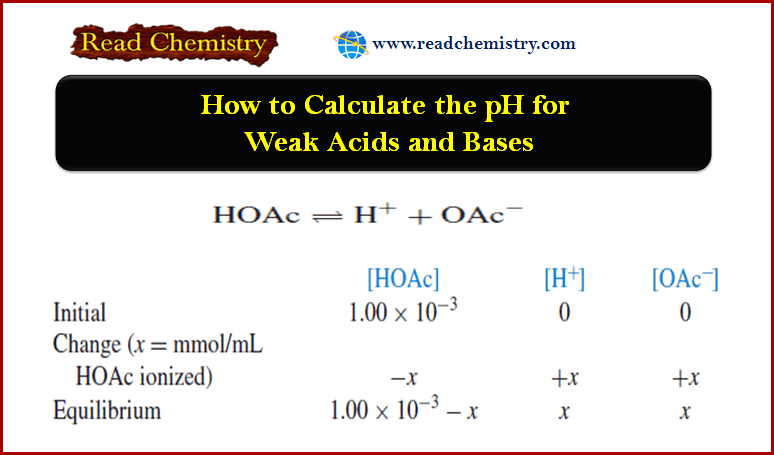
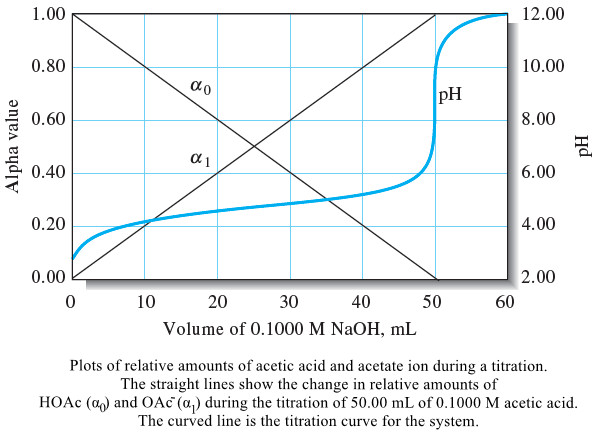
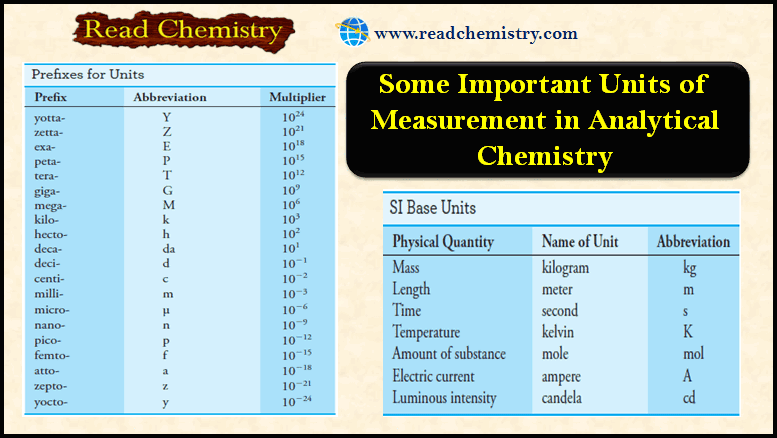
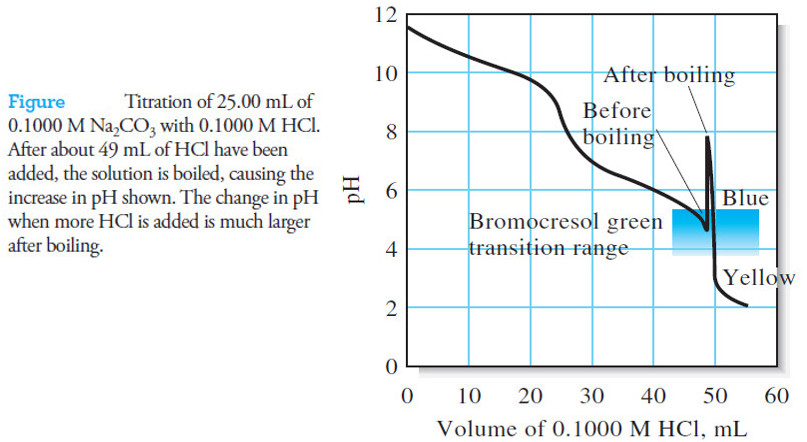
Popular Posts
-
Organic Chemistry
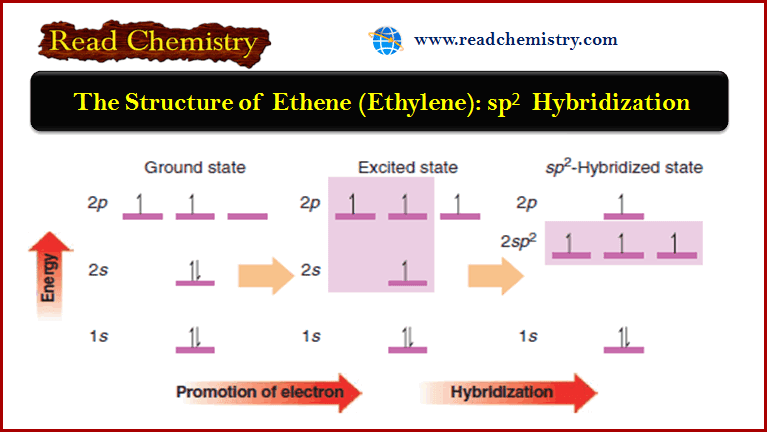
The Structure of Ethene (Ethylene): sp2 Hybridization
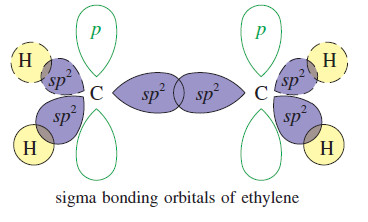
The Structure of Ethene (Ethylene): sp2 Hybridization ** The carbon atoms of many of the molecules that we…
Read More » -
Organic Chemistry
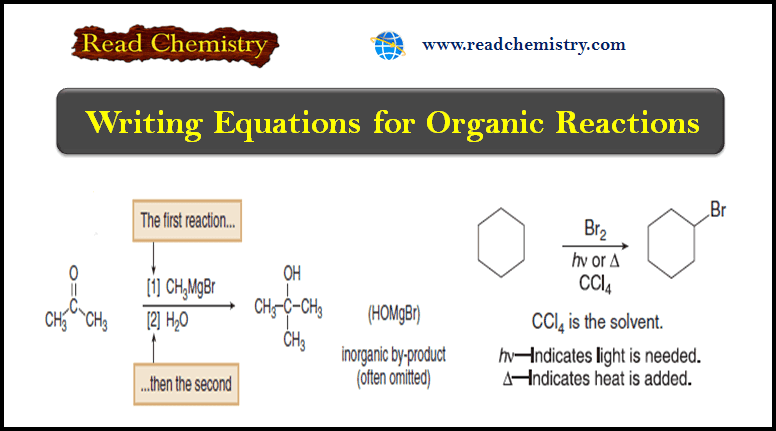
Writing Equations for Organic Reactions
– In this subject, we will discuss Writing Equations for Organic Reactions. Writing Equations for Organic Reactions (1) Like other…
Read More » -
Physical Chemistry
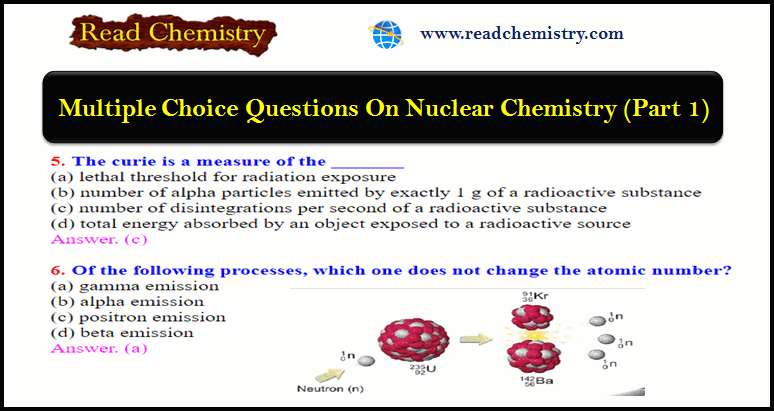
Nuclear Chemistry Quiz: Questions and Answers
Nuclear Chemistry Quiz – In this subject, you will find 40 questions and answers MCQ on Nuclear Chemistry 1. Which…
Read More » -
General Chemistry
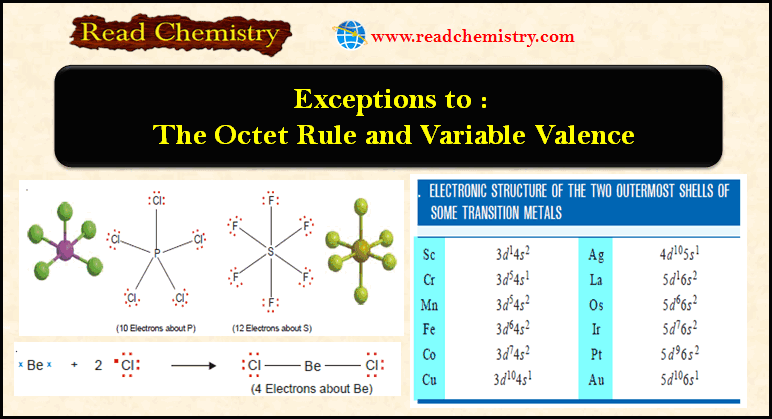
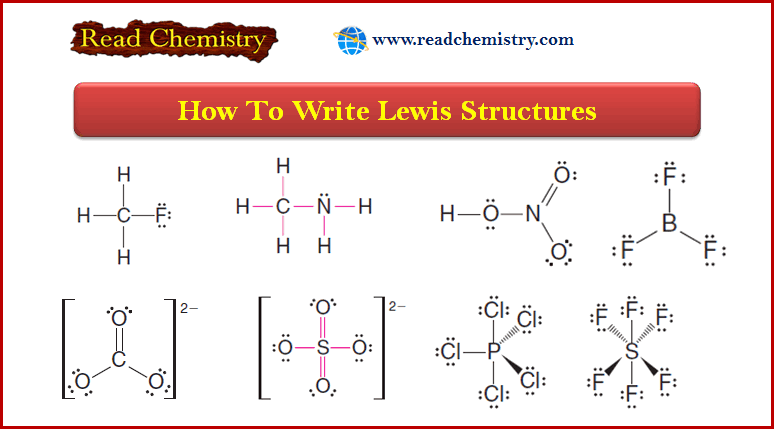
Exceptions to the Octet Rule and Variable Valence
Exceptions to the octet rule – For a time it was believed that all compounds obeyed the Octet rule or…
Read More » -
Physical Chemistry
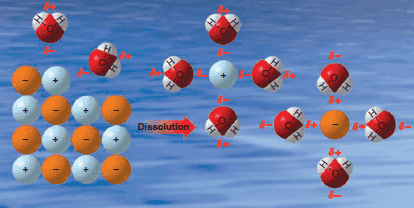
Applications of Colloids
– In this topic, we will discuss The Applications of Colloids. Applications of Colloids – Colloids play an important role…
Read More » -
General Chemistry
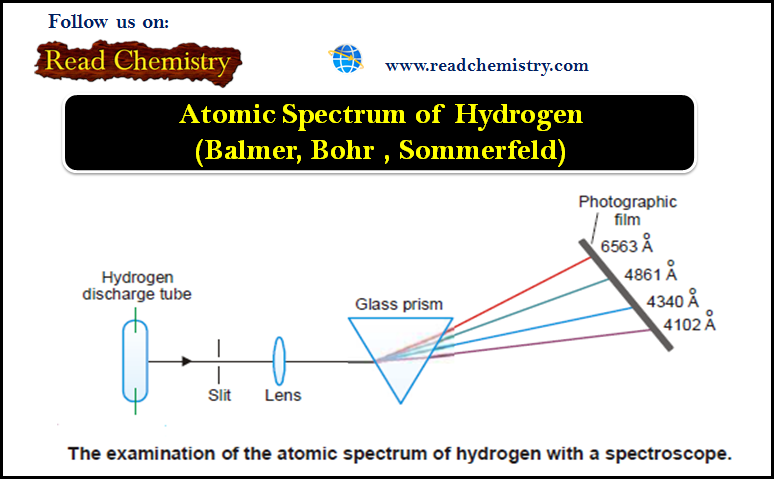
Atomic Spectrum of Hydrogen
– In this subject, we will discuss the atomic Spectrum of Hydrogen Atomic Spectrum – When an element in the…
Read More »
-
Organic Chemistry
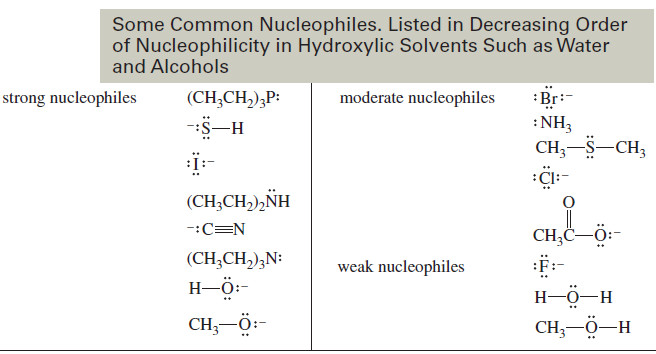
Factors Affecting SN2 Reactions: Strength of the Nucleophile
Factors Affecting SN2 Reactions: Strength of the Nucleophile – we will discuss Factors Affecting SN2…
Read More » -
-
-
-
-
-
-
-
-
-
-
Physical Chemistry
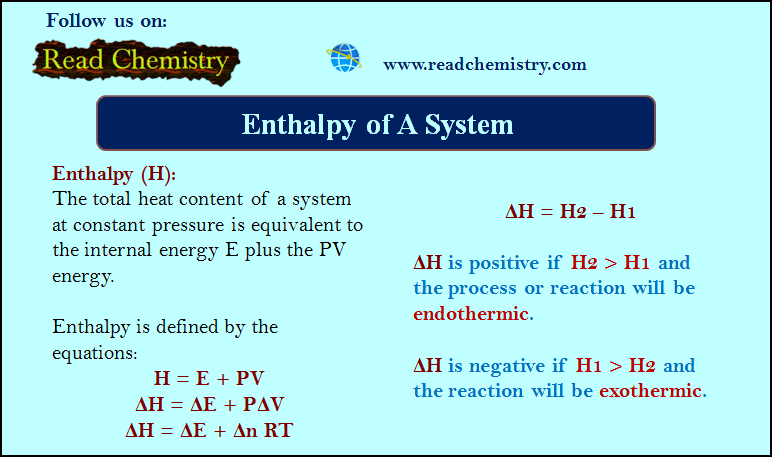
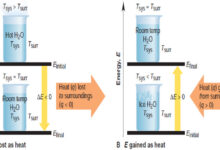
Enthalpy of A System
– Enthalpy (H) is the total heat content of a system at constant pressure and…
Read More » -
-
-
-
-
-
-
-
-
-
-
General Chemistry
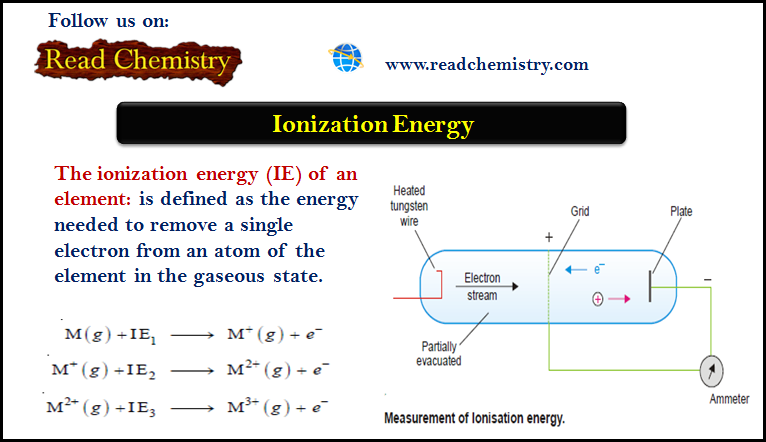
Ionization Energy (Definition – Trends – Measurement)
– The ionization energy (IE) of an element is defined as the energy needed to…
Read More » -
-
-
-
-
-
-
-
-
-
-
Analytical Chemistry
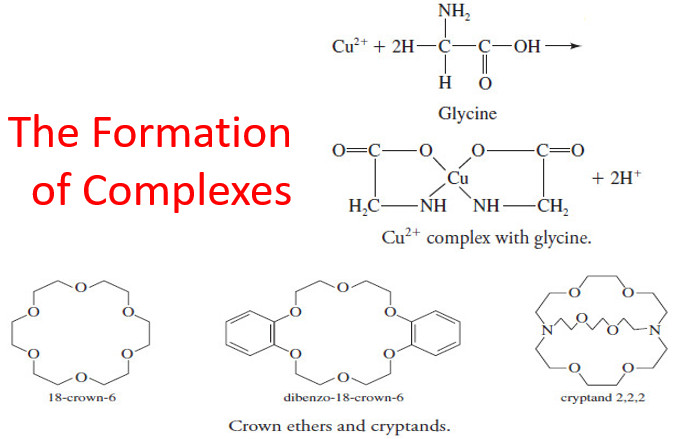
The Formation of Complexes
The Formation of Complexes – Most metal ions react with electron-pair donors to form coordination…
Read More » -
-
-
-
-
-
-
-
-
-
-
Online MCQ
First law of thermodynamics – MCQ online test
Online MCQ test on First law of thermodynamics – In this topic we offer you,…
Read More » -
-
-
-
-
-
-
-
Free book
Physical Chemistry book , 3rd edition by Robert G. Mortimer
– In this subject, we will discuss free download of Physical Chemistry book, 3rd edition…
Read More » -
-
-
-
-